1. Context
The growing prevalence of spinal pain in the United States and across the globe continues to produce substantial economic impact and strain on health-related quality of life. In an assessment in the US Burden of Disease Collaborators for the United States from 1999 to 2010, it was shown that in 2010 low back pain contributed to most years lived with disability (1). The costs of managing spinal pain in the United States, along with its prevalence, continue to increase, ranging up to $100 billion per year, including a wide array of interventions, starting with simple exercises to complex fusions and repeat operations (2-22). Despite various modalities, specifically surgical interventions, disability and economic burden continue to rise across the globe (1, 2, 6, 7, 23).
Pain and disability in the lumbar spine following various types of spinal surgery have been hypothesized to be secondary to multiple causes, including epidural fibrosis, sacroiliac joint pain, disc herniation, discogenic pain, spinal stenosis, arachnoiditis, facet joint pain, and inappropriate surgery (7, 24-32). The development of postoperative epidural fibrosis is a natural process of healing after surgical laminectomy with development of dense scar tissue adjacent to the dura mater (33). This extradural fibrotic tissue may extend into the vertebral canal and adhere to the dura mater and to nerve roots, causing recurrent symptoms, including radicular pain (24, 25, 34, 35). The debate continues with regards to the role of epidural fibrosis as being the major cause of pain after lumbar spine surgery.
Some authors have described a lack of association (26-29). Ross et al. (24) described that patients with extensive epidural fibrosis were 3.2 times more likely to experience recurrent radicular pain in the lumbar spine than those with less scarring. In addition, experimental studies have provided electrophysiological evidence of neurologic disturbances caused by peridural scar formation (36). A multitude of other abnormalities, including mechanical tethering of nerve roots secondary to epidural fibrosis in the vertebral canal (37, 38), disturbances in blood flow (39) and expression of proinflammatory cytokines causing irritation to the exposed dorsal root ganglion and triggering painful responses, have been described (40). Osteopontin also has been shown to play a major role in the formation of epidural fibrosis and a mark-up dorsal root ganglia response to peridural scarring formation (41). Further evidence also has implicated paraspinal muscle spasms, tail contracture, pain behaviors, tactile allodynia, epidural and perineural scarring, and nerve root adherence (42, 43). Additionally, it has been postulated that there may be a final common pathway with all the described etiologies, which results in peripheral and central facilitation potentiated by inflammatory and nerve injury mechanisms (26, 36, 39-44).
Lumbar surgeries for disc herniation, spinal stenosis, degenerative spondylolisthesis, and internal disc disruption have been increasing at a rapid pace (8-12, 45-47). In fact, statistics show an increase in fusions of 131% from 1998 to 2008, whereas laminectomies have not shown a significant increase in the same period (8). Thus, lumbar post-surgery syndrome in patients suffering with multiple symptoms continues to increase. A re-operation rate of 9.5% to 25% at 4 years has been reported, despite advances in surgical techniques (8-10, 40-47). Patients with lumbar post-surgery syndrome not amenable to conservative management, including fluoroscopically directed epidural injections, have been treated with percutaneous epidural adhesiolysis with efficacy and demonstration of cost utility (7, 41, 48-57).
Multiple systematic reviews and guidelines performed by various groups of authors have reached different conclusions about the level of evidence for the effectiveness of percutaneous adhesiolysis (7, 22, 48-51, 58). Some of the authors opined that percutaneous adhesiolysis is not effective in managing lumbar post-surgery syndrome (22, 49, 51). However, other systematic reviews conducted with appropriate methodology showed efficacy based on randomized trials (7, 48, 50), while others have been criticized (2, 51, 58). Thus, the aim of this systematic review is to determine the efficacy of percutaneous adhesiolysis in the lumbar spine, in the treatment of post-surgery syndrome, based on randomized controlled trials.
The objective of this systematic review was to determine the efficacy of all 3 percutaneous adhesiolysis anatomical approaches (caudal, interlaminar, and transforaminal) in treating lumbar post-surgery syndrome.
2. Evidence Acquisition
The methodology utilized in this systematic review followed the widely accepted review process derived from evidence-based systematic reviews and meta-analyses of randomized trials (59-63).
2.1. Data Sources
A literature search was performed from 1966 through October 2014 utilizing data from PubMed, Cochrane library, the US National Guideline Clearinghouse (NGC), previous systematic reviews, and cross references.
2.2. Study Selection
Only randomized controlled trials were utilized, either placebo- or active-controlled. The true definition of placebo is to inject an inactive substance into an inactive structure. For the purposes of this review, we have utilized an injection of placebo into the epidural space or over the nerve root by any approach as placebo, even though it is an impure placebo (64-68). The trials were eligible if the assessment was performed for lumbar post-surgery syndrome. The duration of symptoms of the trial participants was classified chronic if at least 6 months elapsed after surgery. Any of the studies with disc herniation, radiculitis, stenosis, or discogenic pain without previous surgery were not included in this review.
2.3. Data Extraction
The search strategy emphasized post-surgery syndrome and related pathologies treated with percutaneous adhesiolysis procedures. Search terms included lumbar post-surgery syndrome, epidural fibrosis, or lumbar post-laminectomy syndrome, failed back surgery syndrome, adhesions, adhesiolysis, epiduroscopy, hypertonic saline, epidural neuroplasty and epidural scar tissue. Search terminology was as follows:
(((((failed back surgery syndrome) or epidural fibrosis) OR lumbar post-laminectomy syndrome) OR post lumbar surgery syndrome) OR lumbar post-surgery syndrome) and ((((((((((epidural scar tissue) OR epidural adhesions) OR hypertonic saline) OR epidural neuroplasty) OR lysis of adhesions) OR epiduroscopy) OR epidural adhesiolysis) OR adhesiolysis) OR spinal adhesions) OR percutaneous adhesiolysis).
2.4. Outcomes
All studies providing appropriate management and with outcome evaluations of 6 months or longer and statistical evaluations were reviewed.
The primary outcome measure was pain relief. The secondary outcome measure was functional status improvement.
Summary measures included 50% or more reduction in pain in at least 50% of the patients or at least a 3-point decrease in pain scores with an increase in functional status and a relative risk of adverse events including side effects.
2.5. Methodological Quality
The quality of each individual article used in this analysis was assessed by Cochrane review criteria for randomized trials (60) and Interventional Pain Management Techniques–Quality Appraisal of Reliability and Risk of Bias Assessment (IPM-QRB) criteria (61). Only randomized trials meeting the inclusion criteria with at least 4 of 12 Cochrane review criteria or 16 of 48 of IPM–QRB criteria were utilized for analysis. Meta-analysis was considered if more than 2 randomized trials were homogeneous initially with clinical assessment followed by meta-analysis.
At least 2 of the review authors independently, in an unblinded standardized manner, performed each search and methodological quality assessment. The primary authors of manuscripts were not involved in the methodological quality assessment. All searches were combined to obtain a unified strategy. Any disagreements between reviewers were resolved by a third author and consensus.
2.6. Analysis of Evidence
The analysis of evidence was conducted based on the qualitative level of evidence utilizing a modified approach to grading of evidence as shown in Table 1 (62). This was developed from multiple previously utilized grading schemata, most importantly Cochrane reviews and the US Preventive Services Task Force (USPSTF) (7, 63, 69).
| Levels | Description |
|---|---|
| Level I | Evidence obtained from multiple relevant high quality randomized controlled trials |
| Level II | Evidence obtained from at least one relevant high quality randomized controlled trial or multiple relevant moderate or low quality randomized controlled trials |
| Level III | Evidence obtained from at least one relevant moderate or low quality randomized controlled trial with multiple relevant observational studies or Evidence obtained from at least one relevant high quality non-randomized trial or observational study with multiple moderate or low quality observational studies |
| Level IV | Evidence obtained from multiple moderate or low quality relevant observational studies |
| Level V | Opinion or consensus of large group of clinicians and/or scientists |
a Adapted and Modified from: Manchikanti L, Falco FJE, Benyamin RM, Kaye AD, Boswell MV, Hirsch JA. A modified approach to grading of evidence. Pain Physician. 2014; 17: E319 - 25 (62).
Trials were judged to be positive if the injection therapy was clinically relevant and effective, either with a placebo control or active control, with a statistically significant difference in effect for the primary outcome measure at the conventional 5% level. Any improvement of less than 6 months was considered as short-term and 6 months or longer was considered as long-term. Furthermore, the outcomes were judged at the reference point with positive or negative results reported at 3 months, 6 months, and one year.
3. Results
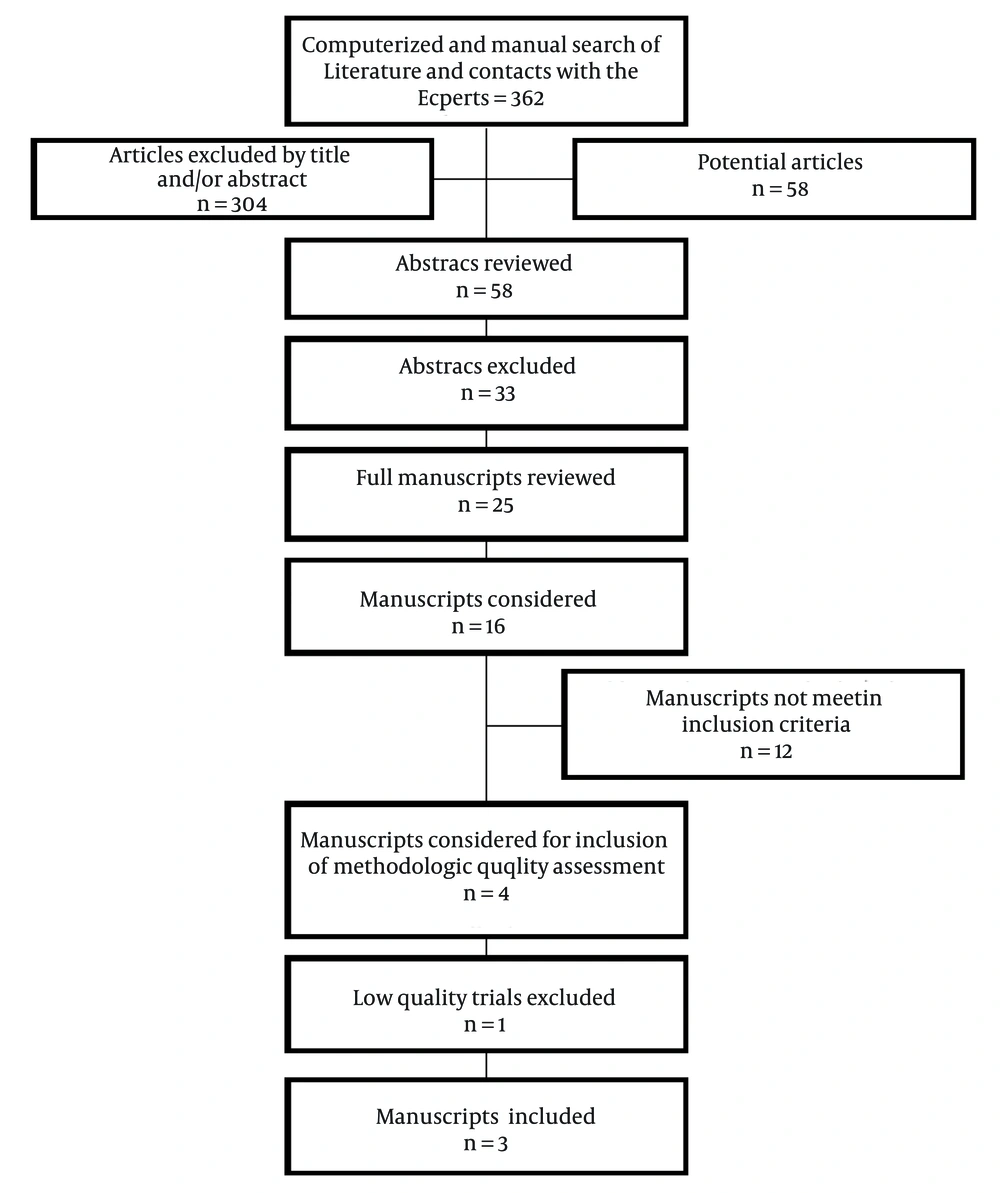
Figure 1 shows a flow diagram of the study selection as recommended by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (59).
Overall, there were 16 manuscripts for consideration (52-57, 70-79). Of these, 4 trials met the inclusion criteria for methodological quality assessment (52, 54, 55, 76). The remaining trials were excluded due to being nonrandomized or assessing various aspects of outcome parameters rather than efficacy. There were no trials utilizing interlaminar or transforaminal approaches.
3.1. Methodological Quality Assessment
The methodological quality assessment of randomized controlled trials is presented in Tables 2 and 3 for randomized trials of percutaneous adhesiolysis.
| Manchikanti et al. (52) | Heavner et al. (54) | Manchikanti et al. (55) | Manchikanti et al. (76) | |
|---|---|---|---|---|
| Randomization adequate | Y | Y | Y | N |
| Concealed treatment allocation | Y | Y | Y | N |
| Patient blinded | Y | Y | Y | N |
| Care provider blinded | N | N | N | N |
| Outcome assessor blinded | N | Y | N | N |
| Drop-out rate described | N | N | N | N |
| All randomized participants analyzed in the group | Y | N | Y | Y |
| Reports of the study free of suggestion of selective outcome reporting | Y | Y | Y | Y |
| Groups similar at baseline regarding most important prognostic indicators | Y | Y | Y | N |
| Co-interventions avoided or similar | Y | Y | Y | N |
| Compliance acceptable in all groups | Y | Y | Y | N |
| Time of outcome assessment in all groups similar | Y | Y | Y | Y |
| Score | 9/12 | 9/12 | 9/12 | 3/12 |
a Source: Furlan AD, Pennick V, Bombardier C, van Tulder Ml; Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009; 34 (18): 1929 – 41) (60).
b Abbreviations: N = No; U = Unclear; Y = Yes.
Among the 4 trials assessed for methodologic quality, there were 3 high-quality trials utilizing Cochrane review criteria as well as IPM-QRB criteria (52, 54, 55). One study (76) was of low quality by Cochrane review criteria and IPM-QRB criteria and was excluded.
3.2. Study Characteristics
Study characteristics of the 3 included trials (52, 54, 55) are shown in Table 4. Of the 3 trials, 2 were performed by Manchikanti et al. (52, 55). The first trial (55) was published in 2004 where a total of 75 patients were divided into 3 groups: Group I with 25 patients randomized to a caudal epidural group with catheterization up to S3 with no adhesiolysis; Group II with 25 patients with adhesiolysis, however, without an injection of hypertonic saline, instead with an injection of 0.9% normal saline; and Group III with 25 patients receiving adhesiolysis, with injection of hypertonic saline. All 3 groups of patients received steroids. Outcome measures were utilized with significant pain relief defined as average relief of 50% or greater. Functional status was assessed with the Oswestry Disability Index (ODI). Results showed significant improvement in patients in Groups II and III at 2 months, 6 months, and 12 months compared to baseline measurements, as well as compared to Group I who did not receive adhesiolysis.
| Variables | Manchikanti et al. (52) | Heavner et al. (54) | Manchikanti et al. (55) | Manchikanti et al. (76) |
|---|---|---|---|---|
| I. Consort or Spirit | ||||
| Trial Design Guidance and Reporting | 3 | 2 | 2 | 0 |
| II. Design Factors | ||||
| Type and Design of Trial | 2 | 2 | 2 | 0 |
| Setting/Physician | 2 | 2 | 2 | 2 |
| Imaging | 3 | 3 | 3 | 0 |
| Sample Size | 3 | 2 | 2 | 0 |
| Statistical Methodology | 1 | 1 | 1 | 1 |
| III. Patient Factors | ||||
| Inclusiveness of Population | 2 | 2 | 2 | 2 |
| Duration of Pain | 2 | 2 | 2 | 2 |
| Previous Treatments | 2 | 2 | 2 | 2 |
| Duration of Follow-up with Appropriate Interventions | 3 | 3 | 3 | 2 |
| IV. Outcomes | ||||
| Outcomes Assessment Criteria for Significant Improvement | 4 | 2 | 4 | 2 |
| Analysis of all Randomized Participants in the Groups | 1 | 0 | 1 | 0 |
| Description of Drop Out Rate | 0 | 0 | 0 | 0 |
| Similarity of Groups at Baseline for Important Prognostic Indicators | 2 | 1 | 2 | 0 |
| Role of Co-Interventions | 1 | 1 | 1 | 0 |
| V. Randomization | ||||
| Method of Randomization | 2 | 2 | 2 | 0 |
| VI. Allocation Concealment | ||||
| Concealed Treatment Allocation | 2 | 2 | 2 | 0 |
| VII. Blinding | ||||
| Patient Blinding | 1 | 1 | 1 | 0 |
| Care Provider Blinding | 0 | 1 | 0 | 0 |
| Outcome Assessor Blinding | 0 | 1 | 1 | 0 |
| VIII. Conflicts OF Interest | ||||
| Funding and Sponsorship | 2 | 2 | 2 | 0 |
| Conflicts of Interest | 3 | 3 | 3 | 0 |
| Total | 41 | 37 | 40 | 13 |
a Source: Manchikanti L, Hirsch JA, Cohen SP, Heavener JE, Falco FJE, Diwan S, et al. Assessment of methodologic quality of randomized trials of interventional techniques: Development of an interventional pain management specific instrument. Pain Physician. 2014; 17 (3): E263-90 (61).
| Study Characteristics Methodological Quality Scoring | Participants/Interventions | Outcome Measures | Pain Relief and Function | Results | Comment(s) | |||
|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | 2 y | |||||
| Manchikanti et al. (52) RA, AC; quality scores: cochrane = 9/12; IPM-QRB = _41/48 | 120; 60 adhesiolysis; 60 caudal epidural; steroid | NRS, ODI, employment status, opioid intake. A significant reduction was 50% for NRS and 40% for ODI. | Caudal = 23%; Adhesiolysis = 78% | Caudal = 7%; Adhesiolysis = 73% | Caudal = 5%; Adhesiolysis = 70% | Caudal = 5%; Adhesiolysis = 82% | 73% of adhesiolysis group had > 50% relief at 12 months; 12% of caudal group did. 3 - 4 adhesiolysis procedures/year | High quality trial showing good evidence of effectiveness. |
| Heavner et al. (54); RA, AC ; quality scores: Cochrane = 9/12; IPM-QRB = 37/48 | 59; 17 Group A: hyaluronidase and hypertonic saline; 15 Group B: hypertonic saline; 17 Group C: isotonic saline; 10 Group D: hyaluronidase and isotonic saline | VAS, MPQ; VAS rated mild (0 - 29), moderate (30 - 54) or severe (55 - 100); Improvement was a 10-point change in VAS. | 40% - 50% of patients improved | 50 - 70% improvement in 3 groups with 20% in normal saline | NA | NA | Significant improvement was seen in 49% at 3 months, 43% at 6 months, and 49% at 12 months. | High quality trial with effectiveness demonstrated with adhesiolysis. |
| Manchikanti et al. (55); RA, AC; quality scores: Cochrane = 9/12; IPM-QRB = 40/48 | 75; 25 caudal epidural steroid injection; 25 1-day adhesiolysis with normal saline; 25 1-day adhesiolysis with hypertonic saline | VAS, ODI, work status, opioid intake, ROM, and psychological evaluation using P-3. Significant pain relief was > 50% relief. | 0%; 64%; 72% | 0%; 60%; 72% | 0%; 60%; 72% | NA | 72% of hypertonic saline and 60% of normal saline patients had > 50% relief at 12 months, versus 0% of caudal injections. | High quality large trial demonstrating efficacy of adhesiolysis with 2-year follow-up and cost utility [57]. |
a Abbreviations: AC = Active Control; MPQ = McGill Pain Questionnaire; NRS = Numeric Rating Scale; ODI = Oswestry Disability Index; P-3 = Pain Patient Profile; RA = Randomized; ROM = range of motion.
Even though patients in Group I achieved improvement, the significant improvement lasted for less than 3 months and at one month some patients showed significant improvement in Group I. At 3 months, 6 months, and 12 months, the improvement in Groups II and III was 64% and 72%, 60% and 72%, and 60% and 72%. Patients received 3 to 4 procedures per year. This was the first trial conducted utilizing a control group. The study has been criticized for the lack of placebo effect with caudal epidural injections (22). Considering that these patients had already failed fluoroscopically directed caudal epidural injections, which was part of the inclusion criteria, along with failure of conservative management, it is not surprising that none of the patients had any significant relief lasting up to 3 months. They did show reductions in their pain scores, as well as disability scores. Additionally, there were a substantial number of patients who withdrew early in the treatment phase in the control group. Only one-fourth of the patients were included through the end of the study. Thus, inappropriate interpretations have led to the impressions that it was a 3 month study even though the majority of the patients were still participating in the trial until 6 months. In the control group an intention-to-treat analysis was utilized. Methodological quality assessment has taken into consideration the withdrawals of greater than 20% with an assigned score of “0”.
The second trial by Manchikanti et al. (52) also studied the role of percutaneous adhesiolysis compared with caudal epidural injections. They randomized 120 patients into 2 groups with a control group of 60 patients receiving caudal epidural injections with catheterization up to S3 with injection of local anesthetic, betamethasone, and 0.9% sodium chloride solution. In contrast, the intervention group of 60 patients received percutaneous adhesiolysis, followed by injection of 10% hypertonic sodium chloride solution, and nonparticulate betamethasone. Robust outcome measures were utilized with a follow-up lasting up to 24 months with the primary outcome defined as 50% improvement in pain and ODI scores. The treatments were repeated as pain returned and disability ensued. Overall, patients received 6 to 7 procedures over a period of 2 years in Group II with approximately 78 weeks of relief out of 104 weeks. In this trial, significant improvement was seen in 82% of patients at 2 year follow-up in the intervention group compared to 5% in the control group receiving caudal epidural injections. In contrast to the previous trial (55), in this trial, 23% of the patients showed significant improvement at 3 months, 7% at 6 months, and 5% at 12 months and 24 months. Considering the protocol and the design of the trial, the majority of the patients (62%) were unblinded at the end of one year in the control group, whereas only 3% were unblinded in the treatment group. However, an appropriate intent-to-treat analysis was performed. The trial may be criticized for not using a placebo group; however, a placebo group is extremely difficult in interventional pain trials. Consequently, the control group with caudal epidural injections seemed to be the most appropriate. Methodological quality assessment has taken into consideration the issue related to withdrawals greater than 20%.
The third trial by Heavner et al. (54) included 59 patients assigned randomly into 4 treatment groups: Group A with 17 patients received hyaluronidase and hypertonic saline, Group B with 15 patents received hypertonic saline, Group C with 17 patients received isotonic saline, and Group D with 10 patients received hyaluronidase and isotonic saline. They concluded that percutaneous epidural neuroplasty, as part of an overall pain management strategy, reduced pain in at least 25% or more of patients with radiculopathy plus low back pain refractory to conventional therapies.
Compared to trials by Manchikanti et al. (52, 55) which were one-day procedures, Heavner et al. (54) published the trial in 1999 and utilized a 3-day protocol. The study has been criticized for its lack of a control group with all patients undergoing adhesiolysis even though one group received isotonic saline instead of hypertonic saline or hyaluronidase. The results showed a lack of significant improvement with the combination of hypertonic saline and hyaluronidase. However, patients receiving either hypertonic saline, normal saline, or hyaluronidase faired equally. Overall, 83% of the patients showed some improvement; however, significant improvement was seen in 49% at 3 months, 43% at 6 months, and 49% at 12 months. Any deficiencies in this trial were taken into consideration during methodological quality assessment.
3.3. Meta-Analysis
Three included trials were considered for meta-analysis. There was no homogeneity among the trials. Hence, a meta-analysis was not feasible.
3.4. Analysis of Evidence
The results of randomized trials of the efficacy of epidural injections and percutaneous adhesiolysis are shown in Table 4. Based on the qualitative best evidence synthesis and based on 3 relevant high quality, randomized controlled trials, the evidence for percutaneous adhesiolysis in managing lumbar post-surgery syndrome is Level II.
4. Conclusions
This systematic review of randomized controlled trials of percutaneous adhesiolysis in managing lumbar post-surgery syndrome showed evidence of Level II based on 3 relevant high quality randomized controlled trials (52, 54, 55) showing the efficacy of percutaneous adhesiolysis for long-term improvement. All the patients included in these trials suffered from chronic low back pain after surgical interventions. After the failure of multiple modalities of conservative management, including fluoroscopically directed epidural injections, the trials were conducted appropriately and were shown to be of high quality based on Cochrane review criteria as well as IPM-QRB criteria. These trials showed improvement in pain and functional status.
The evidence in this systematic review, while similar to some systematic reviews previously published (7, 48), does not correlate with other reviews (22, 49, 51). The systematic review by Helm et al. (48) was performed appropriately and showed the efficacy of adhesiolysis. However, other reviews were mostly of either a narrative nature or utilized inappropriate methodology (49, 51). These reviews have been criticized for their methodology (22, 51, 58). Chou and Huffman (22) misinterpreted the results and provided inaccurate analysis, and reached inappropriate conclusions (58). In fact, Chou and Huffman (22) not only provided inaccurate data, but also utilized the control group which received caudal epidural injections in this trial to demonstrate the failure of caudal epidural injections which is inappropriate as these patients already had failed caudal epidural injections prior to their inclusion into the adhesiolysis group.
The surgical interventions described in managing post-surgery syndrome have shown only modest results (10-13, 22, 25, 78, 79); however, spinal cord stimulation has shown clinical efficacy and cost effectiveness in multiple trials of post-surgery syndrome (80, 81). Caudal epidural injections (82), as well as percutaneous adhesiolysis (57) have shown cost utility in managing lumbar post-surgery syndrome. Cost utility in post-surgery syndrome was assessed (57) based on a randomized trial (54) utilized in this assessment which showed cost utility at $2,650 per Quality-Adjusted Life-Year (QALY). Thus, percutaneous adhesiolysis is a viable option in managing post-surgery syndrome patients after the failure of conservative management including caudal epidural injections prior to proceeding with spinal cord stimulation or repeat surgery.
Percutaneous adhesiolysis, a procedure designed to lyse epidural scarring in patients with persistent low back and leg pain due to lumbar post-surgery syndrome, has evolved over the years with a changing definition and concepts. It was originally described as a 3-day procedure using hypertonic saline, local anesthetic, steroid, and hyaluronidase administered using a reinforceable catheter. Heavner et al. (54) showed that neither hypertonic saline nor hyaluronidase was critical for a successful outcome. Subsequently, Manchikanti et al. (55) studied the efficacy of one-day percutaneous adhesiolysis. Two (52, 55) of the 3 trials (52, 54, 55) included in this assessment were based on one-day adhesiolysis, whereas one trial (54) was based on 3-day percutaneous adhesiolysis. Patients received approximately 3 to 4 interventions during one year and 6 interventions during a 2-year period with one-day adhesiolysis whereas with the 3-day adhesiolysis by Heavner et al. (54), only one procedure was carried out. Consequently, the results must be interpreted cautiously in comparing these trials.
The major issues related to conducting research pertaining to percutaneous adhesiolysis, along with other interventional techniques, involve the control group, placebo effect, and nocebo effects. Placebo control neural blockade is not only unrealistic, but also provides inaccurate results. Further, some have interpreted any local anesthetic injection yielding similar results as steroids as placebo. The evolving literature on placebo, nocebo, and the effect of inactive solutions when injected into active structures has been extensively discussed in recent years (64-68). Methodologists tend to focus on the difference between 2 groups, ignoring equivalency trials and non-inferiority trials, as well as the basis of comparative effectiveness research, which essentially evaluates the differences or similarities between 2 treatments. Thus, conclusions that neither treatment works is inappropriate. Instead, it should be that both treatments work and there is no difference. In fact, in a recent widely publicized trial by Friedly et al. (83) assessing outcomes of interlaminar and transforaminal epidural injections in managing spinal stenosis, the authors misinterpreted the evidence by assigning 2 different levels of evidence synthesis for combined analysis with a P value of 0.05 and changing it to a P value of 0.025 for interlaminar and transforaminal epidural injections when analyzed separately (84). In essence, this trial showed the efficacy of local anesthetic, as well as steroids, for 6 weeks following one procedure in the majority of the patients, which is appropriate as the effect of epidural injections with the first injection lasts approximately 3 weeks on average (7, 85-87). While the design of placebo is an extremely difficult venture in interventional pain management, specifically with percutaneous adhesiolysis, recently 2 appropriately designed trials have been published (67, 68).
Percutaneous adhesiolysis involves multiple components of treatment with adhesiolysis, injection of local anesthetic, steroid, hypertonic sodium chloride solution, and hyaluronidase. All the components have not been applied in all the trials. All the components have not been well studied separately. These components provide different mechanisms of action and result in variable outcomes in chronic, persistent recalcitrant pain secondary to post surgery syndrome. Corticosteroids have been shown to be anti-inflammatory, along with local anesthetics (7, 52, 54, 55, 85-87). The hypertonic saline utilized in percutaneous adhesiolysis has been shown to attenuate the transmitter release from an exposed neuromuscular junction (7, 52, 54, 55, 85-87). Other mechanisms also included C fiber blockade in cat dorsal rootlets with an increased concentration of chloride ion, decrease of the spinal cord water content, and depressed lateral column evoked ventral root response change in the volume due to outflow of water across the membrane and ionic concentration changes, reduction and swelling are by osmotically induced fluid shifts, reducing pressure on the nerve, and reduce local anesthetic effect of hypertonic solution (67).
The results of this systematic review are clinically oriented and may be applied in interventional pain management practices utilizing appropriate evaluation. Future implications for research should include a clear case definition with consistent inclusion and exclusion criteria; technical considerations; frequency, type, and volume of injectate; appropriate design and outcome measures; and compliance with CONSORT guidelines.
In conclusion, this systematic review provides practical evidence for management of an extremely difficult problem with recalcitrant low back and lower extremity pain secondary to lumbar post-surgery syndrome with 3 relevant high-quality randomized controlled trials providing qualitative evidence of Level II.