1. Background
Spinal neuraxial blocks result in sympathetic blockade, sensory analgesia, or anesthesia and motor blockade, depending on the dose, concentration or volume of local anesthetic, after insertion of a needle in subarachnoid space. Despite similarities of various local anesthetics used for spinal anesthesia, there are significant physiologic and pharmacologic differences. Local anesthetics provide longer and better quality of block when used in adjunct to the additive drugs such as opioids. The adjunct drug should be utterly controllable in blocking motor and lacking systemic side effects (1).
Addiction is an increasing problem in modern society and affects patients’ management in anesthesia. It is well recognized that the prolonged use of opioids is associated with a requirement for ever-increasing doses in order to maintain pain relief at an acceptable and consistent level. Addicted patients have innate tolerance to local anesthetics in both neuraxial and peripheral blocks (2). Dexmedetomidine has better effects on sensory and motor block duration and motor block onset in comparison with ketorolac, as lidocaine adjuvants in infraclavicular brachial plexus block (3).
Dexmedetomidine (DEX), a highly selective α2 adrenergic receptor agonist, is a newly discovered drug that gained much reputation in neuroanesthesia, intensive care unit (ICU) and cardiac anesthesia in recent years (4). DEX has eight times α2/α1 activity compared with clonidine (5); therefore, it is considered a full agonist of the α2 receptor. Compared to clonidine, DEX has proposed unique features to maintain analgesia, anxiolytic and sedative effect without causing major respiratory depression in this sense. Notably, DEX was suggested as an additive to local anesthetics in peripheral and neuraxial blocks (6, 7) in many previous studies. Although available data are insufficient, DEX is a good local anesthetic (LA) adjuvant that can hasten the onset and prolong the duration of sensory and motor blockade when used in intrathecal or epidural block and looks safe (8). Some previous studies showed that α2 adrenergic receptor agonist prolongs the effects of local anesthetics (9-11), however these results are mixed (12, 13).
2. Objectives
The current study aimed to compare the effect of dexmedetomidine and fentanyl additives on bupivacaine to prolong the duration of block and minimizing side effects.
3. Patients and Methods
This study was a triple blinded randomized clinical trial (RCT) of 84 patients randomly divided into three groups of 28 patients. This study was performed in Loghman hospital, Tehran, Iran; January 2013 - January 2014.
Inclusion criteria were the American society of anesthesiologists (ASA) class I patients aged 18 to 60-years-old and elective surgery less than three hours for lower abdomen or lower extremities surgeries. Exclusion criteria were history of uncontrolled hypertension, allergy to bupivacaine, fentanyl, or dexmedetomidine, contraindication for spinal anesthesia, or failed spinal.
3.1. Patient Selection
Patients were randomly allocated to receive dexmedetomidine (Precedex, Hospira, USA) 5 µg diluted in 0.5 mL normal saline added to 12.5 mg (2.5 mL) of 0.5% hyperbaric bupivacaine (Mylan, Italy) (DEX group) or 25 µg (0.5 mL) fentanyl (Caspian Tamin, Iran) added to 12.5 mg (2.5 mL) of 0.5% hyperbaric bupivacaine (F group). Control group received 0.5 mL normal saline added to 12.5 mg of 0.5% hyperbaric bupivacaine. Randomization was performed using accidental numbers with concealed allocation. Anesthesiologists administrating drugs and anesthesiologists evaluating level and duration of sensory block were blinded to the syringe content.
3.2. Ethics Declaration
The study was reviewed and approved by the Shahid Beheshti University of Medical Science Ethics Committee. Information about the study was given comprehensively both orally and in written form to all patients or their accompanying adults. They gave their informed written consents prior to their inclusion in the study according to University Ethical Committee.
3.3. Method of Spinal Anesthesia
After entrance to operation room, patients were monitored for all standard procedures including oxygen saturation (pulse oximetry), electrocardiography (ECG), non-invasive blood pressure and heart rate. Then 500 mL Ringer was infused. The patient was positioned in sitting position and spinal anesthesia was performed by midline approach in L3-L4 level with a Quincke 25 G needle. The palpating fingers (usually the index and third fingers) identified the interspinous area by locating the caudad extent of the more cephalad spine and the midline by rolling the fingers in a medial to lateral direction. After inserting the needle into the subarachnoid space and obtaining clear cerebrospinal fluid (CSF), the drug of study was injected after careful aspiration. After injection, patients were immediately aligned into supine position and 5 L/min oxygen was provided through face mask. This moment was assumed as baseline time point and all vital signs were recorded.
3.4. Data Entry
Data were recorded based on the sensory block using pin prink sensory test. Motor block was tested using modified Bromage scale every 30 minutes until the end of block. Time to return of sensory block to 4 dermatomes below and time to return of Bromage scale to 0 were recorded.
All vital measurements, including SPO2, heart rate (HR), electrocardiography (ECG) and noninvasive blood pressure amplifier (NIBP), were performed at 0, 30, 60, 90, 120 and 180 minutes in all three groups of the study. All data were recorded in a data sheet specified to each patient.
All intra-operative and post-operative adverse effects of spinal anesthesia including post-operative nausea and vomiting (PONV), pruritus and respiratory depression were measured. Post-operative pain score were measured using visual analogue scale (VAS) score scaled from 0 to 10, as 0 being the least and 10 the worst. Post operation pain scales were measured at 1, 3, 6, and 24 hours after injection. In case of VAS ≥ 4 the patients received morphine as rescue analgesic until VAS ≤ 3. Sedation scale was measured by Ramsay sedation scale at 0, 1, 2 and 3 hours after intrathecal injection during and after anesthesia.
3.5. Statistical Analysis
Statistical calculations were conducted using SPSS 20 (Chicago, IL, USA). The parametric variables were presented as Mean ± SD and analyzed by the analysis of variance (one-way ANOVA) post-hoc test; non-parametric variables were analyzed by Kruskal-Wallis test. P < 0.05 was considered statistically significant. Sample size was estimated using sample size calculator software with 95% confidence interval, P = 0.05 and power of 80% and difference between the two groups of 30% in primary outcome based on pilot study.
4. Results
Totally, 84 patients were randomly divided into three groups of 28 patients. There was no significant difference in age, gender and body mass index (BMI, kg/m2) of patients between the three groups (P > 0.05) (Table 1). Duration of surgery was 150 ± 44 minutes in the DEX group, 152 ± 41 minutes in the fentanyl group and 147 ± 42 minutes in the control group. Duration of surgery was not significantly different between the three groups (P > 0.05) (Table 1).
| Variable | DEX | Fentanyl | Marcaine b |
|---|---|---|---|
| Age, y | 45.7 ± 11.3 | 44.5 ± 15.8 | 46.9 ± 13.6 |
| Weight, kg | 65.7 ± 13.8 | 69.8 ± 16.3 | 64.7 ± 17.8 |
| BMI, kg/m2 | 22.4 ± 3.8 | 23.8 ± 4.4 | 21.6 ± 3.9 |
| Duration of surgery, min | 150 ± 44 | 152 ± 41 | 147 ± 42 |
aP > 0.05
bBupivacaine is the control group.
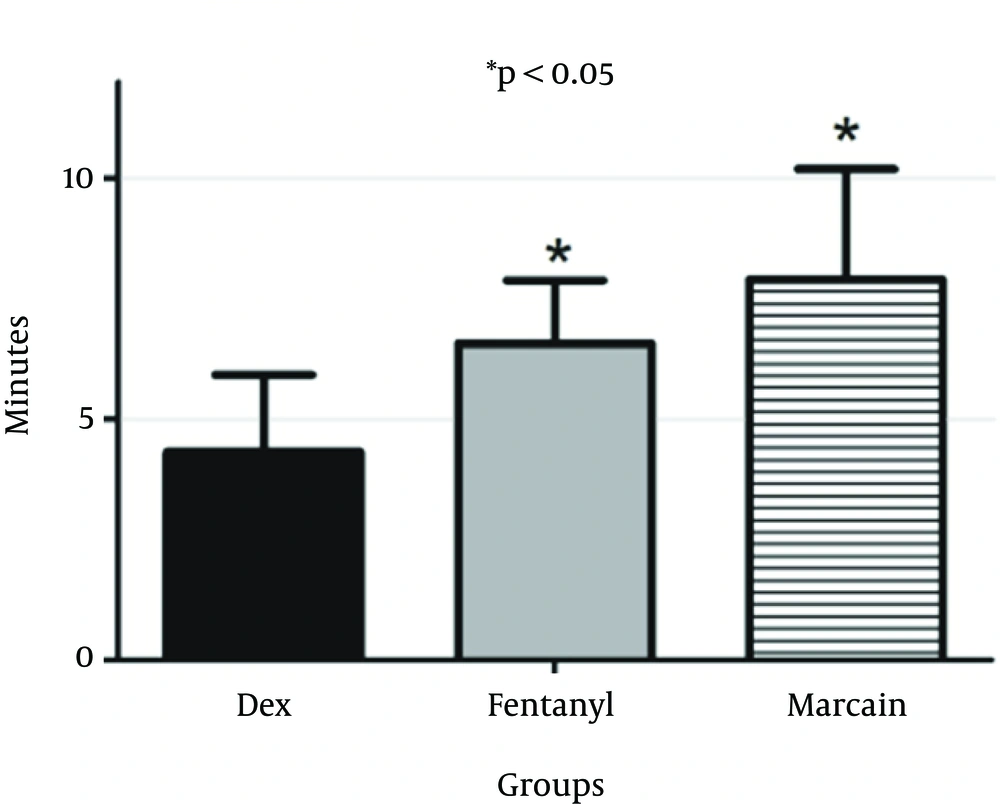
Mean of onset of sensory block in the DEX group was 4.32 ± 1.6 minutes, 6.58 ± 1.3 minutes in the F group and 7.9 ± 2.3 minutes in the control group. Onset of sensory block in DEX group was significantly lower than the fentanyl (P = 0.012) and control groups (P = 0.001) (Figure 1). Besides, onset of sensory block was significantly lower in the fentanyl group than the control (P = 0.035) (Marcaine).
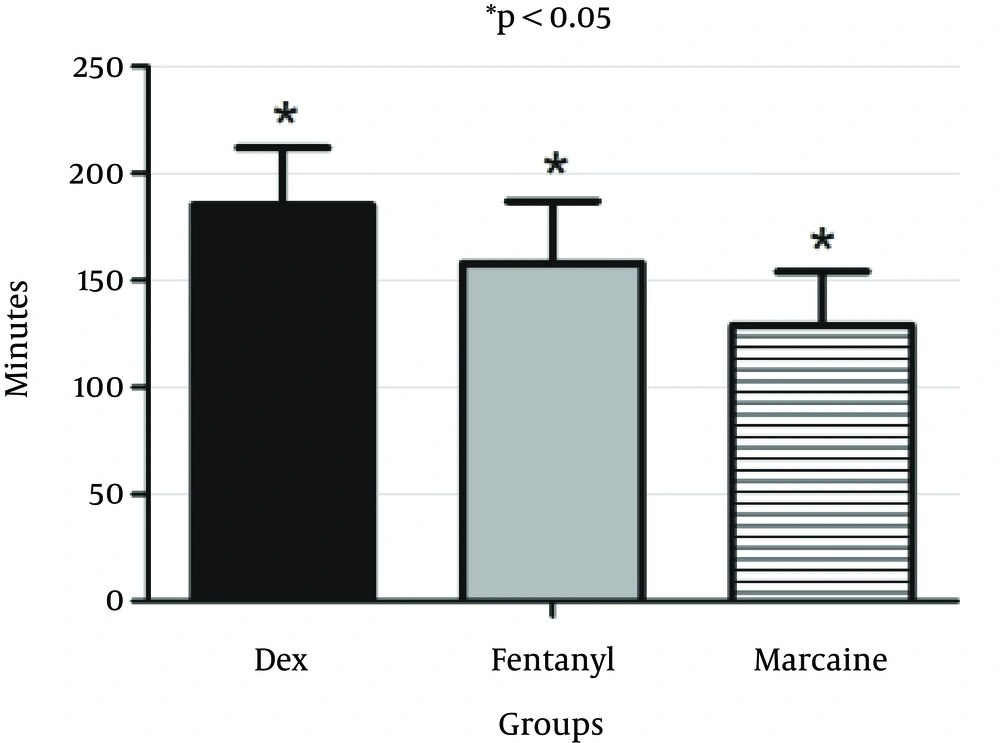
Duration of sensory block was 185.5 ± 27.5 minutes in the DEX group, 158.5 ± 29.7 minutes in the fentanyl group and 129.3 ± 25.4 minutes in the control group. Duration of sensory block was significantly longer in the DEX group compared to the fentanyl (P = 0.043) and control (P = 0.016) (Figure 2). Duration of sensory block was significantly longer in the fentanyl compared to the control group (P = 0.024) (Marcaine).
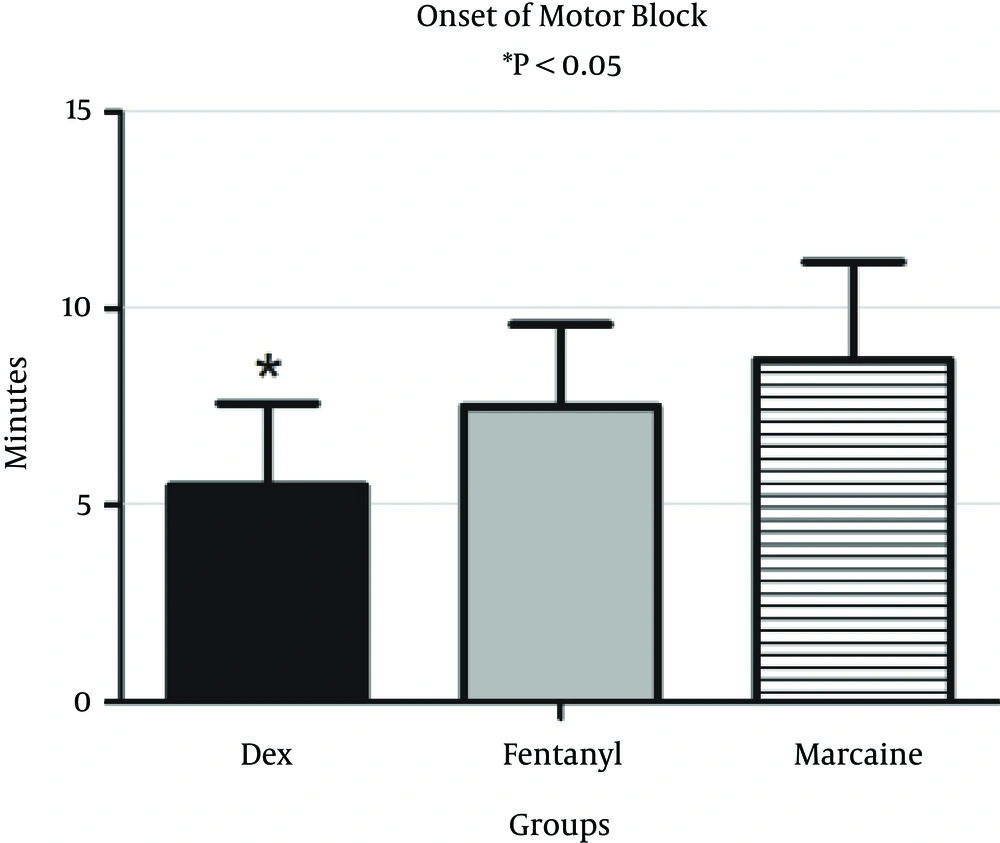
Onset of motor block in the DEX group was 5.46 ± 2.6 minutes, 7.57 ± 2.3 minutes in the F group and 8.69 ± 2.16 minutes in the control group. Onset of motor block in the DEX group was significantly lower than those of the fentanyl (P = 0.024) and control groups (P = 0.022) (Figure 3). Onset of motor block was not significantly lower in the fentanyl group than the control (P = 0.073).
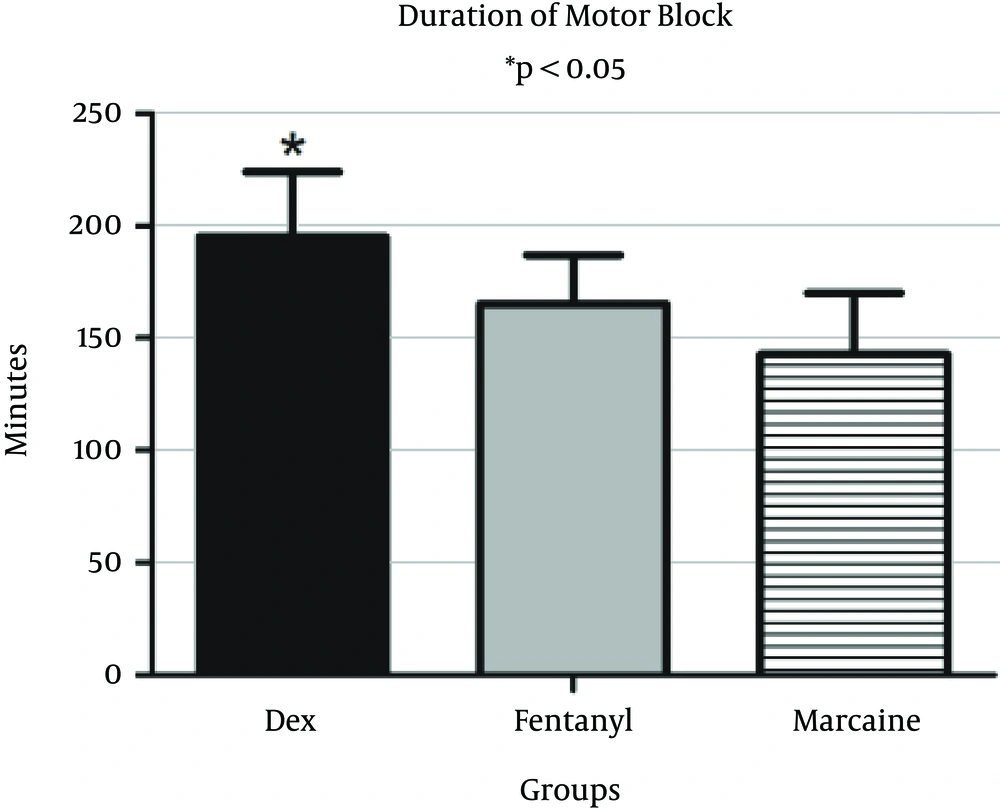
Duration of motor block was 196.5 ± 24.5 minutes in the DEX group, 165.5 ± 22.7 minutes in the F group and 143.9 ± 22.1 minutes in the control group. Duration of motor block in the DEX group was significantly longer than those of the fentanyl (P = 0.014) and control groups (P = 0.005) (Figure 4). Duration of motor block was not significantly lower in the fentanyl group than the control (P = 0.081).
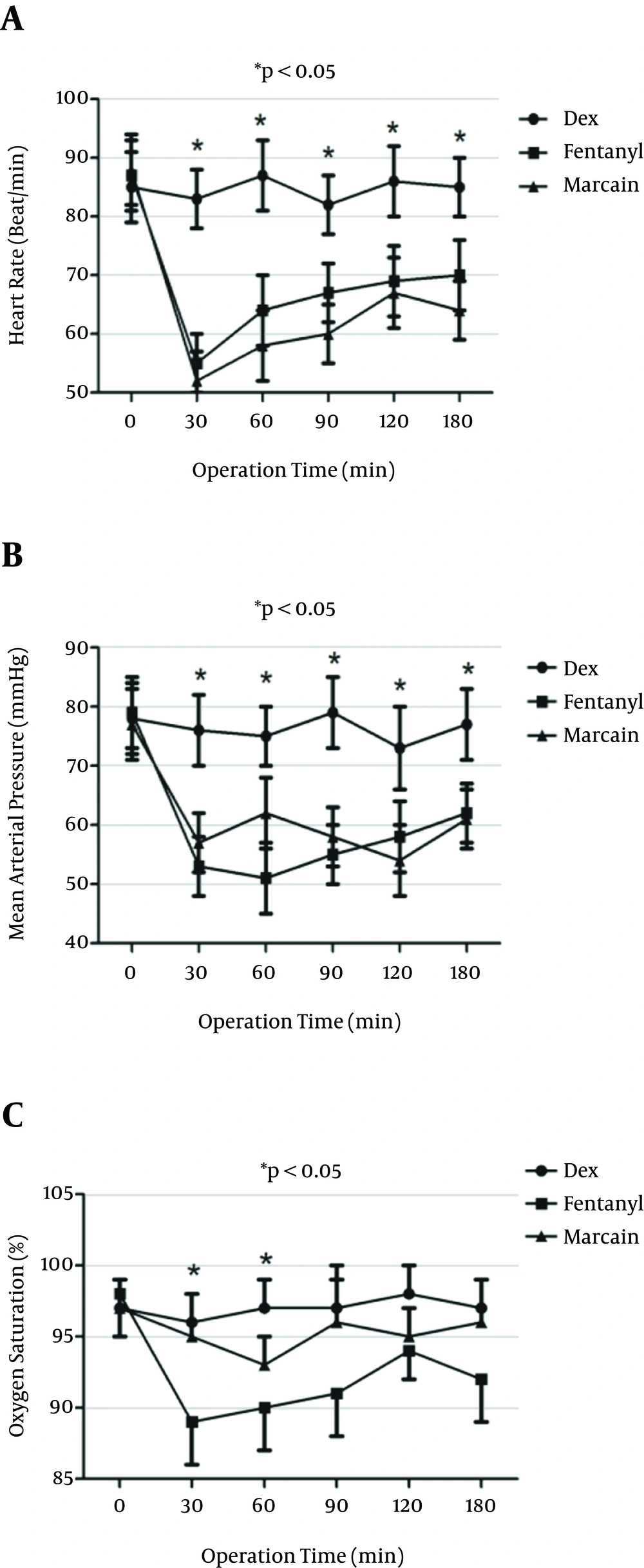
Heart rate was depicted in the three groups of the study during operation. Heart rate was significantly higher in the DEX group at 30, 60, 90,120, and 180 minutes compared to the other two groups (P < 0.05) (Figure 5). Mean blood pressure was also significantly higher in the DEX group compared to the fentanyl and control groups at 30, 60, 90, 120 and 180 minutes compared to the fentanyl and control groups (P < 0.05) (Figure 5). Arterial oxygen saturation was significantly higher in the DEX group as compared to the fentanyl and control groups at 30 and 60 minutes (P < 0.05).
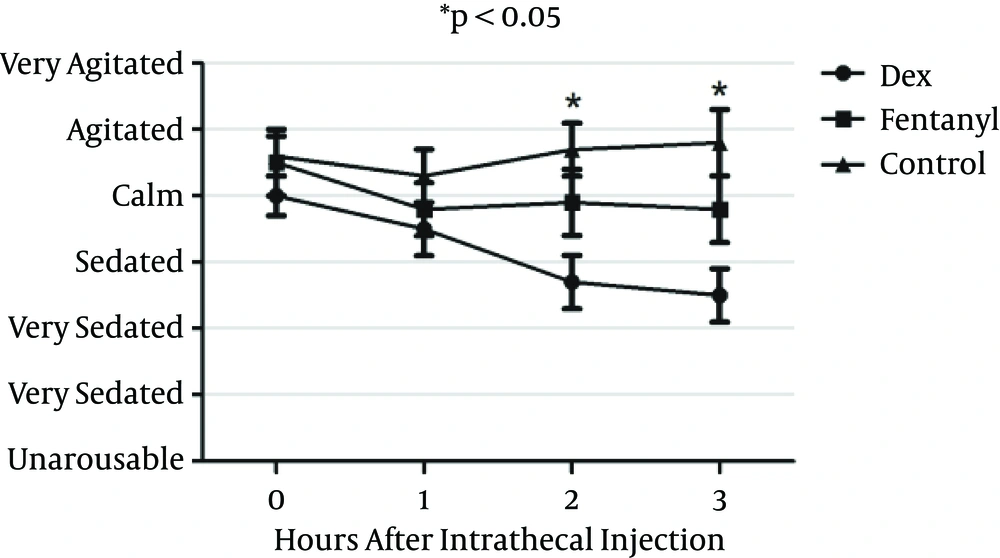
Sedation scale was measured using Ramsay sedation score at zero, one, two and three hours after intrathecal injection. Sedation score was significantly better in the DEX group compared to the fentanyl and control groups (P < 0.05) in two and three hours but not in zero and one hours (P > 0.05) (Figure 6).
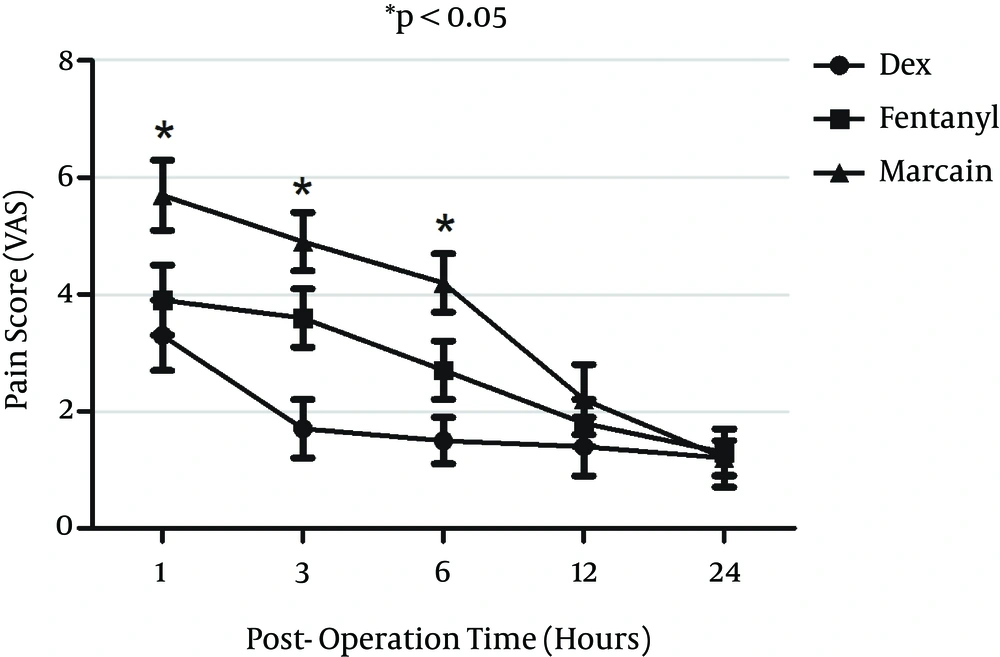
Post-operative pain score was measured using visual analogue scale (VAS) at one, three, six and 24 hours after surgery. VAS scores in the DEX group at three and six hours post operation time were significantly lower than those of the fentanyl group (P < 0.05); Besides, at one, three and six hours, VAS scores in the DEX group were significantly lower than those of the control group (P < 0.05). VAS scores in the Fentanyl group at one, three and six hours were significantly lower than those of the control group (P < 0.05) (Figure 7).
Total morphine requirement dose in the DEX group was significantly lower than those of the fentanyl and control groups at zero and one hour (Table 2). However, at 12 hour post-operation morphine requirement was not significantly lower in the DEX group compared to fentanyl and control groups (P > 0.05) (Table 2). Total morphine dose in 24 hours was significantly lower in the DEX group as compared to fentanyl and control groups (P < 0.05) (Table 2).
Incidence of post-operative nausea and vomiting (PONV) in the DEX, fentanyl and control groups were 14.5%, 38.5% and 22.5%, respectively (Mann-Whitney). PONV was significantly lower in the DEX group compared to fentanyl (P = 0.03) and control groups (P = 0.022) (Table 2).
aBupivacaine is the control group.
bANOVA post-hoc test: DEX vs. Fentanyl: P = 0.02; DEX vs. Marcaine: P = 0.001.
cANOVA post hoc-test: DEX vs. Fentanyl: P = 0.003; DEX vs. Marcaine: P = 0.015.
dANOVA post-hoc test: DEX vs. Fentanyl: P = 0.001; DEX vs. Marcaine: P = 0.002.
5. Discussion
The current study attempted to compare dexmedetomidine (DEX) and fentanyl as the appropriate adjunct drugs for spinal block in addicted patients. Addicted patients may need higher doses of bupivacaine in combination with an adjunct drug. The results showed that an adjunct dose of dexmedetomidine significantly enhanced onset and duration of block, stabilized vital signs and provided more appropriate sedation; besides it decreased post operation pain, post operation morphine requirement and the incidence of PONV compared to addition of fentanyl to bupivacaine or bupivacaine alone.
The current study results showed a substantial effect of DEX adjunct to bupivacaine in shortening the onset of block and increasing the duration of block compared to those of the fentanyl additive or bupivacaine alone. Although the exact mechanism of DEX additive is not clear in intrathecal injection, however its strong and significant effect on improving the quality and quantity of spinal block in addicted patients suggests a direct anesthetic action of this drug. Zhang et al. attempted to find intrathecal action site for DEX (14), they suggested a direct action for DEX on motor neurons or posterior horn neurons or their synapses in spinothalamic pathway (15). The second theory was that DEX increases duration of block through indirect action. It means that α2 agonist activation affect other receptors inside spinal canal such as Na-K exchange channels and acetylcholine receptors (16, 17). Another mechanism also suggested for DEX including antinociception and vasoconstriction (18). Significant effect of DEX on shortening onset and increasing duration of neuraxial block could be the same as its effect on increasing peripheral nerve block duration. On the other hand, intrathecal additives of opioids gained much popularity (19), due to their significant effects on increasing the duration of blocks in addicted patients. However, the current study showed superiority of intrathecal DEX to fentanyl when used in addicted patients, consistent with other studies (20).
Pain and sedation scales were improved in the DEX patients compared to those of fentanyl and control groups. Patients in the DEX group were mostly calm and sedated even better than the ones in the fentanyl group. Sedative effects of systemic DEX were proved in many previous studies, but the current study showed significant sedative effects of DEX when used in intrathecal injections. Better sedation scale could be the result of both anxiolytic and analgesic effects of intrathecal DEX simultaneously. Sedative effects of intrathecal DEX could be mediated by interacting with its receptors in locus coeruleus through circulation of cerebrospinal fluid (CSF) (21).
Opium sparing effect is of particular importance in addicted patients due to less withdrawal symptoms and side effects. Besides, patients in the DEX group showed lower pain scores which could result in less withdrawal and agitation symptoms after surgery, consistent with other studies (22, 23). Opium sparing effects of dexmedetomidine was also one of the important aspects of the current study. Opium addicted patients have tolerance and dependence to opium. Morphine doses were significantly lower in the DEX group compared to midazolam group. In addition, α2 agonists such as clonidine and DEX are useful to alleviate withdrawal symptoms in addicts and controlling postoperative pain when added to morphine pumps (24).
Patients’ hemodynamics remained more stable in the DEX group compared to those of the midazolam group, which gives advantage to DEX in these patients. DEX has sympatholytic effects (25) and hypothetically could depress blood pressure and heart rate. However, patients in the DEX group had significantly higher blood pressure and heart rate, which was consistent with other studies (26). This is of particular importance while systemic DEX could induce profound hypotension and bradycardia. The current study used adjusted intrathecal dose of DEX which could be a complete different entity. In addition, oxygen saturation and respiratory rate were higher in the DEX group compared to the fentanyl and control groups, as expected.
One other interesting result of the study showed that DEX decreased PONV incidence as compared to intrathecal fentanyl. This is of particular importance in both addicted and non-addicted patients. Intrathecal fentanyl induces PONV as a major side effect in many patients (27).
No adverse neurologic problems were observed in any of the patients; however longer follow-ups and experimental studies could determine all adverse neurologic deficits. Future studies are needed to further explore the exact mechanism of intrathecal fentanyl and dexmedetomidine site of action and neurologic pathway, particularly in addicted patients.
In conclusion, dexmedetomidine added to bupivacaine in spinal anesthesia was more effective to increase the duration of block, provide more appropriate sedation and less postoperative pain scale and PONV compared to fentanyl additive.