1. Background
Neurologic complications after surgery are postoperative cognitive dysfunction (POCD) and postoperative delirium (POD). Geriatric patients with cognitive dysfunction can usually show poor nutritional status and delayed wound healing. Delayed physical and emotional rehabilitation due to cognitive dysfunction may complicate the recovery after a surgery.
POCD is relevant and important in the geriatric surgical population. POCD, defined as surgery-related deterioration in cognition, can be detected days to months after surgery (1). There are no standardized methods to score the testing batteries and determine cognitive dysfunction within the POCD literature. POD is a change of postoperative mental status characterized by a reduced awareness of the environment and a disturbance in attention (1). Diagnostic and statistical manual of mental disorders (DSM-IV) criteria or confusion assessment method (short-CAM) are used to diagnose POD. POCD is more difficult to define than POD. To identify POCD, patients are given a battery of neuropsychological tests including attention, memory, executive function, and visuospatial function before and after surgery.
There is an association between the occurrence of POCD and increased mortality in the first year after major non-cardiac surgery (2). POCD is reported in 53% of patients at discharge from hospital, 36% at six weeks, and 24% at 6 months after coronary artery bypass graft surgery (3). Also, it is reported in total hip arthroplasty and minor surgery in early postoperative phase and the first 24 hours after surgery (4, 5).
Risk factors of POCD are advancing age, operative complications, second operation, duration of anesthesia (> 2 hours), level of education and intraoperative transfusion (6, 7). Advancing age is a major risk factor for POCD. Also, increased inflammatory activity may contribute to cognitive dysfunction in elderly patients (7). C-reactive protein (CRP) is a sensitive marker of systemic low-grade inflammation (8). High serum CRP is a useful biomarker to detect increased risk of cognitive decline and is associated with poorer memory at 12-year follow up (9, 10). Therefore, CRP was used as a biomarker of systemic inflammation and cognitive decline.
Ketamine, an N-methyl D-aspartic acid (NMDA) receptor antagonist is a traditional intraoperative anesthetic agent. It displays neuroprotective effects including the prevention of excitotoxic injury and apoptosis. Suppression of central nervous system (CNS) inflammatory response might be responsible. A single dose of ketamine (0.5 mg/kg) attenuates POD and POCD with an anti-inflammatory effect after cardiopulmonary bypass (11, 12). But, the effects of ketamine on early postoperative cognitive function after a non-cardiac surgery are unclear.
2. Objectives
The current study aimed to evaluate the effect of single dose ketamine (0.5 mg/kg) on early postoperative cognitive function in elderly patients undergoing non-cardiac surgery.
3. Patients and Methods
This prospective, randomized, double-blind study was approved by the institutional review board of Haeundae Paik hospital, Busan, Republic of Korea (2012 - 064). Written informed consent was obtained from all of the patients and the neurocognitive function tests were explained to them before surgery. This study protocol conformed to the ethical guidelines of the 1975 Helsinki declaration.
Fifty six elderly patients (> 60-years-old) with the American society of anesthesiologist physical status 1 - 3, scheduled to undergo orthopedic surgery (duration of anesthesia > two hours) were recruited. Exclusion criteria were CNS-related diseases (including dementia), medication with tranquilizers or antidepressants, inability to perform neurocognitive function tests, severe visual or auditory dysfunction, alcohol abuse or drug dependence, and a history of a cerebrovascular accident within the previous three years.
Patients were randomly divided into two groups using a computer-generated randomization program (www.random.org): K group received intravenous (IV) bolus, a total of 3 mL mixed with 0.9% normal saline and 0.5 mg/kg ketamine (50 mg/mL, Huons, Seongnam, Korea), and N group received IV 0.9% normal saline (3 mL). The patients enrolled in the present study were not premedicated because preoperative medications including benzodiazepine and anticholinergics can cause POCD (6).
Upon arrival in the operating room, the patients were monitored by electrocardiogram (ECG), non-invasive blood pressure, and pulse oximetry. After applying standard monitor to the patients, ketamine and 0.9% normal saline were administrated by a nurse who was blinded into the study before induction of anesthesia. General anesthesia was induced with IV propofol 2 mg/kg and rocuronium 0.6 mg/kg, maintained with desflurane 5 - 6 vol%, medical air (FiO2 0.5), and remifentanil 0.02 - 0.1 mcg/kg/hour. Remifentanil was titrated due to clinical needs (hemodynamic, autonomic, and somatic signs) depending on stimulus of surgery. Bispectral index (BIS, aspect medical system, Norwood, MA, USA) remained at 40 - 60 during surgery. End-tidal carbon dioxide concentration remained at 35 - 45 mmHg using continuous gas analysis equipment (Datex-Ohmeda S/5; Datex-Ohmeda Inc. Louisville, CO). The esophageal temperature (DeRoyal Inc. Powell, TN, USA) was measured through the surgery and maintained at 35.5 - 36.5°C. On completion of the operation, the administration of desflurane was discontinued and the fraction of inspired oxygen was increased to 100%. To reverse the neuromuscular block, IV pyridostigmine 10 mg and IV glycopyrrolate 0.2 mg were administered. Extubation was performed when the patient obeyed commands and recovered regular self-breathing.
Intravenous patient-controlled analgesia (IV-PCA) or intermittent bolus IV analgesics were used for postoperative pain targeting a visual analog scale of 2 - 3 depending on the type of surgery. The IV PCA solution contained fentanyl, ketorolac, and 3 mg of ramosetron mixed with 100 mL normal saline. The continuous infusion rate was set at 2 mL/hour, the bolus at 2 mL, and the lockout time at 15 minutes. The initial dose of fentanyl was 15 - 20 mcg/kg and that of ketorolac was 2 - 3 mg/kg, depending on the physical status and body weight of the patient as well as the surgical procedure the patient underwent. In patients not using IV PCA, meperidine, fentanyl, and ketorolac were used as rescue analgesics.
Neurocognitive function tests were conducted by a trained investigator who was blinded into the study before surgery (baseline test) and on postoperative day one (POD 1) and postoperative day six (POD 6) in a quiet environment. Serum CRP concentrations were measured.
Patients had cognitive dysfunction when the two Z-scores in more than two tests or the combined Z-score was 1.96 or more. In addition, the following factors were recorded and compared between the two groups. Duration of anesthesia and surgery, frequency of transfusion, and ketamine-related complications (psychosis or hemodynamic changes) were also recorded.
It was estimated that 28 patients in each group were needed to show a difference in POCD between 47% and 7% (5), assuming a type I error of 0.05, a type II error of 0.2 and a 10% drop-out rate.
Statistical analyses were performed by SPSS version 21.0 (SPSS Inc. Chicago, IL, USA) and Medcalc 11.6.1.0 (Medcalc software bvba, Ostend, Belgium). The data were expressed as mean ± standard deviation (SD), median (interquartile range) or number of patients. The chi-square test was used to compare categorical variables, and the independent t test or Mann-Whitney U test was used to compare continuous variables between the K and N groups. An analysis of variance with repeated measures was performed to determine the difference between the groups and over time. P value < 0.05 was considered statistically significant.
4. Results
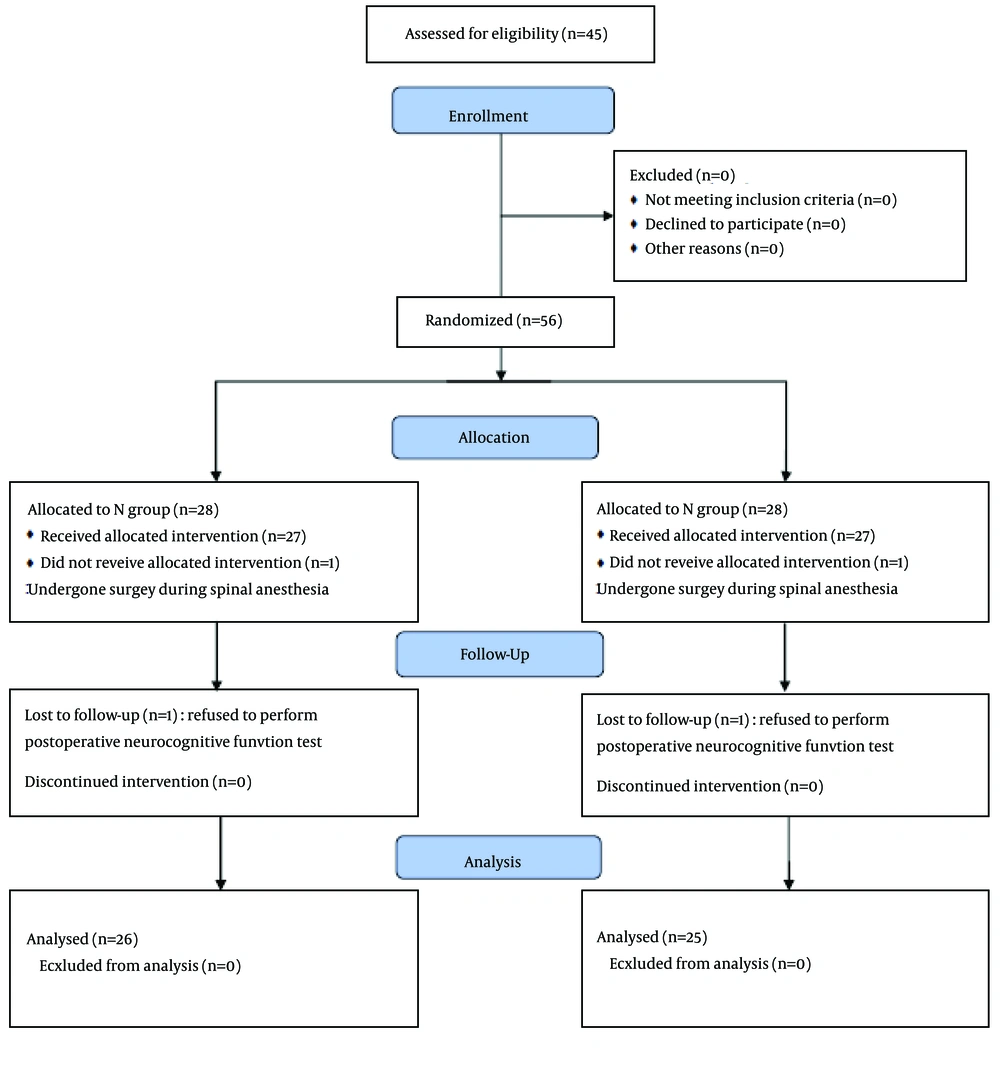
Of the fifty six patients enrolled in this study, five patients were excluded. Three patients refused to perform postoperative neurocognitive function tests and two had undergone operation during spinal or local anesthesia (Figure 1).
The two groups had similar demographic characteristics except for gender (Table 1). There were more female patients in N group randomized of the patients. Surgical and anesthetic data were also similar (Table 2).
| Patients' Characteristics | N (n = 26) | K (n = 25) | P Value |
|---|---|---|---|
| Age, y | 68.38 ± 6.54 | 68.32 ± 5.34 | 0.97 |
| Gender | 0.02 | ||
| Male | 5 | 14 | |
| Female | 21 | 11 | |
| ASA | 0.46 | ||
| 1 | 3 | 1 | |
| 2 | 16 | 19 | |
| 3 | 7 | 5 | |
| BMI, kg/m2 | 26.11 ± 4.69 | 25.43 ± 2.36 | 0.51 |
| Alcohol | 0.30 | ||
| Yes | 5 | 9 | |
| No | 12 | 16 | |
| Smoking | 0.96 | ||
| Yes | 3 | 2 | |
| No | 23 | 23 | |
| Education level, n | 0.29 | ||
| Un-education | 4 | 1 | |
| Elementary school | 7 | 10 | |
| Middle school | 7 | 6 | |
| High school | 7 | 4 | |
| University | 1 | 4 | |
| MMSE, units | 26.5, (24.0 - 28.0) | 25.0, (24.0 - 28.0) | 0.66 |
| TMT, s | 73.5, (45.5 - 103.0) | 59.0, (43.25 - 105.0) | 0.71 |
| DST, units | 24.5, (16.0 - 40.0) | 31.0, (19.0 - 44.25) | 0.41 |
| CRP, mg/dL | 0.18, (0.08 - 0.28) | 0.10, (0.06 - 0.26) | 0.20 |
| Surgical and Anesthetic Characteristics | N (n = 26) | K (n = 25) | P Value |
|---|---|---|---|
| Type of surgery, n | 0.82 | ||
| Acromioplasty | 9 | 12 | |
| CTS | 1 | 1 | |
| ORIF | 3 | 1 | |
| Spine surgery | 5 | 4 | |
| THRA | 1 | 2 | |
| TKRA | 7 | 5 | |
| Transfusion | 0.70 | ||
| Yes | 3 | 3 | |
| No | 23 | 22 | |
| Anesthetic time, min | 187.31 ± 73.69 | 195.60 ± 72.19 | 0.69 |
| Operation time, min | 125.19 ± 70.00 | 139.60 ± 67.85 | 0.46 |
The results of change from baseline in neurocognitive function tests and CRP concentration are shown in Table 3. There was a statistically significant difference between the trail-making test scores. Trail-making test score increased more in the N group (52.5 points) than the K group (13 points) at POD 1 (P = 0.047) compared with baseline scores. There were no significant differences in the mini-mental status examination, digit substitution test, and CRP concentration between the two groups. But, a trend toward decreased cognitive function was evident at POD 1 but neurocognitive function tests returned to baseline at POD 6. There were no significant differences in the mini-mental status examination (P = 0.19), trail-making test (P = 0.08), digit substitution test scores (P = 0.28), and concentration of CRP (P = 0.59) between the two groups throughout the time.
| Variables | N | K | P Value |
|---|---|---|---|
| MMSE, units | |||
| Baseline | 26.5, (24.0 - 28.0) | 25.0, (24.0 - 28.0) | 0.66 |
| ΔPOD1 | -2, (-4.0 - 0.0) | -2, (-3.25 - -0.75) | 0.98 |
| ΔPOD6 | 0, (0 - 1) | 0, (-1 - 0) | 0.33 |
| TMT, s | |||
| Baseline | 73.5, (45.5 - 103.0) | 59.0, (43.25 - 105.0) | 0.71 |
| ΔPOD1 | 52.5, (9.0 - 118.0) | 13, (0 - 52.5) | 0.047 |
| ΔPOD6 | 8, (1 - 30) | 1, (-4.25 - 17) | 0.089 |
| DST, units | |||
| Baseline | 24.5, (16.0 - 40.0) | 31.0, (19.0 - 44.25) | 0.41 |
| ΔPOD1 | -9.5, (-15 - -3) | -5, (-13 - -2.25) | 0.39 |
| ΔPOD6 | -2.5, (-7.0 - 1) | -3, (-5.25 - 0.25) | 0.81 |
| CRP, mg/dL | |||
| Baseline | 0.18, (0.08 - 0.28) | 0.10, (0.06 - 0.26) | 0.20 |
| ΔPOD1 | 2.67, (0.43 - 10.96) | 3.41, (1.44 - 11.82) | 0.55 |
| ΔPOD6 | 1.91, (0.44 - 6.01) | 2.42, (1.25 - 6.95) | 0.39 |
POCD (the two Z-scores in more than two tests or the combined Z-score was 1.96 or more) occurred in one patient (4%) in K group at POD 6 (P = 0.98). Only one patient had a Z-score > 1.96 on both mini-mental status examination and trail-making test in K group at POD6. There were no ketamine-related complications such as psychosis or hemodynamic changes.
5. Discussion
The current study evaluated the effect of ketamine on early postoperative cognitive function after orthopedic surgery undergoing desflurane anesthesia in the elderly. The incidence of POCD was 0% at POD 1 in both groups, and 4% at POD 6 in K group. The obtained results did not show any statistical differences between two groups. The power was calculated considering a difference of 40% of POCD incidence. However, a difference of 10% - 15% might be clinically more realistic and this study might be underpowered.
Previous studies considered different outcome measures of early cognitive function in the elderly after general anesthesia. There are some possible reasons for the negative findings in the primary outcome. Old age is a major significant risk factor of POCD (2, 13, 14). However, Hosseini et al. reported that age alone is not a strong predictor of complications (15). Preoperative health status such as diabetes mellitus, hypertension, glomerular filtration rate, and left ventricular ejection fraction are also major contribution factors. A bolus dose (0.5 mg/kg) of ketamine was used because this dose proved to reduce systemic inflammation, POD, and POCD associated with cardiopulmonary bypass (11, 12). After cardiac surgery, cognitive function tests decreased by at least two SDs (Z-score of 1.96) in 21 of 26 patients in the placebo group. Only 7 of 26 patients in the ketamine group showed cognitive decline (12). Ketamine reduces post-ischemic neuronal cell loss in the cortex associated with glutamate-calcium overload and has a preconditioning-like effect through the temporary inactivation of NMDA receptors. The high incidence of POCD after coronary artery bypass surgery may be related to the surgical procedure (> eight hours), cardiopulmonary bypass (> two hours), microembolism, or intraoperative transfusion (11, 12). This may have reflected the absence of microemboli, temperature changes, or hypoperfusion during cardiopulmonary bypass in the current study.
The current study results were different from those of Rohan et al. who studied the incidence of POCD 24 hours after minor surgery using Stroop test and Modified Word Recall test in the elderly (> 65-year-of-age) (5). Different methods are used to measure and define POCD, and they did not check the depth of anesthesia. General anesthesia was maintained with desflurane 5 - 6 vol% targeting BIS 40 - 60. A significant decrease in postoperative POCD by limiting anesthetic exposure when BIS was maintained between 40 and 60 during surgery is reported (16). Administration of desflurane or sevoflurane titrated to moderate general anesthesia as guided by processed electroencephalography (patient state index 25 - 50) was not associated with a lasting decrease in MMSE (17).
A major methodological issue in the study of POCD is lack of uniformly accepted battery of neurocognitive tests and no general consensus established regarding the optimum timing of assessments after surgery. Trail-making test is used to assess attention, concentration, perception and visuospatial function (18). In The current study, trail-making test results showed a significant difference compared with preoperative scores. Green et al. also, reported that trail-making test showed statistically significant differences in comparison with pre and post exposure of desflurane (19). Digit substitution test measures the speed of general information processing (20). When neuropsychological tests were first introduced into the study of POCD, MMSE was assessed as a screening test (21). MMSE is a 30-item measure of global cognitive function. Postler et al. also reported 6.7% of POCD at one week after surgery using MMSE (4).
Volatile agents themselves are implicated as causative factors in cognitive decline after surgery. Desflurane is widely used volatile anesthetic that is characterized with a low blood-gas partition coefficient. The total incidence of POCD was similar at 6 - 8 hours and 66 - 72 hours between the desflurane and the sevoflurane groups (22). POCD (> 65-years-old), defined as 20% decline in 20% of the cognitive function tests (the paper-pencil tests and the cognitive test for attentional performance) were detected 36% - 47% in both anesthetics. But, Z-score was used to determine POCD.
Female gender and low educational level contributed to POCD (20). Females are high risk patients for neurologic complications associated with mild atherosclerosis of the ascending aorta, higher prevalence of vascular disease of cerebral arteries, duration of cardiopulmonary bypass (CPB) and aortic cross-clamping, and length of hospitalization after cardiac surgery (23). Although there were more females and uneducated patients in N group, no correlations were found between gender, concentrations of CRP and incidence of POCD.
Intensive postoperative pain control might reduce the occurrence of POCD in both groups. Cognitive function assessments during early postoperative period can be influenced by postoperative pain, analgesic therapy and physical disability (1). Pain is a major contributor to cognitive dysfunction and effective strategies to reduce pain should be adopted (24). Although adequate analgesics were used for postoperative pain targeting a visual analog scale of 2 - 3 depending on the type of surgery, wide variability of surgery can be a limitation source of the current study.
Although there was no significantly different incidence of POCD in the study, POCD may occur in non-cardiac surgery. Treatment and prevention are important. Treatment is aimed at early recognition, supportive care and education of family members. To prevent POCD, use of long-acting benzodiazepine must be avoided and surgical time should be shortened. Excessive perioperative fasting should also be avoided and proper analgesic techniques should be provided to reduce the stress response after surgery. Also, early discharge from hospital and family member’s education are essential.
The incidence of POCD was not significantly influenced by a bolus dose of ketamine (0.5 mg/kg) after orthopedic surgery in elderly patients. Also, there was no negative effect of ketamine on early POCD. Neurocognitive function tests were performed six days after surgery. Further studies will be required to assess long-term results.