1. Introduction
Postoperative traumatic lesions to the spinal cord can be particularly challenging to manage. Spinal cord injury (SCI) presents complex motor, sensory and autonomic dysfunctions with symptom severity often associated with the extent of the damage, and for which no effective therapy has been developed (1, 2). Various neuro-protective and neuro-generative pharmaceutical agents have been studied in laboratory and clinical settings to treat SCI. After injury to central nervous system, neuro-protective pharmaceutical treatments are needed to minimize secondary damage and enhance repair. Erythropoietin (EPO) and its non-erythropoietic derivatives, asialo-EPO and carbamylated-EPO have shown some promise in preclinical laboratory animal models of SCI (3).
We illustrate a case where recovery after cervical spinal cord injury was considerably improved with concurrent administration of erythropoietin (EPO).
2. Case Presentation
Informed patient consent was obtained for the presentation of this case. A 42-year-old female patient presented with gait instability and progressive weakness in her lower limbs, over a 6-year period. She did not have any history of trauma or falls, complaints of coordination or sphincter difficulties. She was known for dyslipidemia and breast augmentation surgery, and smoked one pack of cigarettes per day. She was clinically myelopathic with diffuse hyperreflexia, clonus, and Hoffman’s sign bilaterally. She had decreased sensation to light touch over the left C6 dermatome. Her muscle strength was Medical Research Council grade (MRC) 5/5 in all muscle groups in upper and lower limbs and her gait was normal with down going toes bilaterally. Magnetic resonance imaging (MRI) demonstrated severe spinal stenosis at the C4-5 and C5-6 levels with myelomalacia.
The patient underwent a C4-5 and C5-6 anterior cervical discectomy and fusion (ACDF) through a standard right-sided Smith-Robinson approach assisted by fluoroscopy without any complications. In the post-anesthesia care unit (PACU), the patient was noted to develop decreased strength in her right hand side (MRC grade 3/5 and 1/5 in proximal and distal muscle groups of the right upper limb and 0/5 in all muscle groups of the right lower limb), along with left-sided hypoesthesia, consistent with a clinical diagnosis of Brown-Sequard syndrome at the cervical level consistent with the criteria for American Spinal Cord Injury Association (ASIA) grade D spinal cord injury (SCI) at a cervical level. Urgent imaging revealed increased myelomalacia/edema and potential epidural hematoma. The patient was returned the same day to the operating room for a C4-6 posterior laminectomy and instrumented fusion. A standard approach to laminectomy was taken, and polyaxial lateral mass screws were placed bilaterally in the lateral masses of C4, C5, and C6, and fixed to a metal rod. Her strength, however, did not improve immediately postoperatively.
On her second postoperative day, she complained of profuse neuropathic pain in her left and right limbs which progressed to the neck in subsequent days. She was started on pregabalin along with opioid analgesia without adequate pain control.
The pain management service evaluated patient on the sixth postoperative day; she suffered from right hemiplegia and right hemi-paresthesia, severe abdominal distention, constipation, and urinary retention. Lidocaine 75 mg/hour IV, N-acetyl-cysteine 600 mg PO BID, ketamine 5 mg PO BID, and low dose naloxone 1 mg PO BID were started by the pain service. The signs and symptoms of patient’s did not change the next day. To decrease the inflammation and for neuron protection and with the patient's consent for an off-label treatment, darbepoetin alpha (Aranesp) 100 mcg subcutaneous daily for three days, was subsequently added to her medications. The next day, after the first dose of erythropoietin treatment, she felt better, the severity of pain was decreased and abdominal distention, and urinary retention resided.
During the next week, she slowly recovered strength in her lower limbs. On 17th day after surgery, she was discharged for inpatient rehabilitation with MRC grade 3/5 strength in her lower limbs. At her 2-month follow up, strength was 3+/5 in her lower limbs bilaterally. At 8 months, she was ambulating with a cane, and after one year she walked without help and returned to her previous job.
3. Discussion
Erythropoietin (EPO) is a glycoprotein hormone that has been employed clinically in the treatment of anaemia (4). It was documented that the central nervous system (CNS) also produces erythropoietin, and expression is thought to be regulated by various factors including hypoxia (4, 5). Erythropoietin has been shown to have protective actions in various preclinical in vitro and in vivo models of injuries and other diseases associated with neurodegenerative diseases and neuronal death (3, 6, 7).
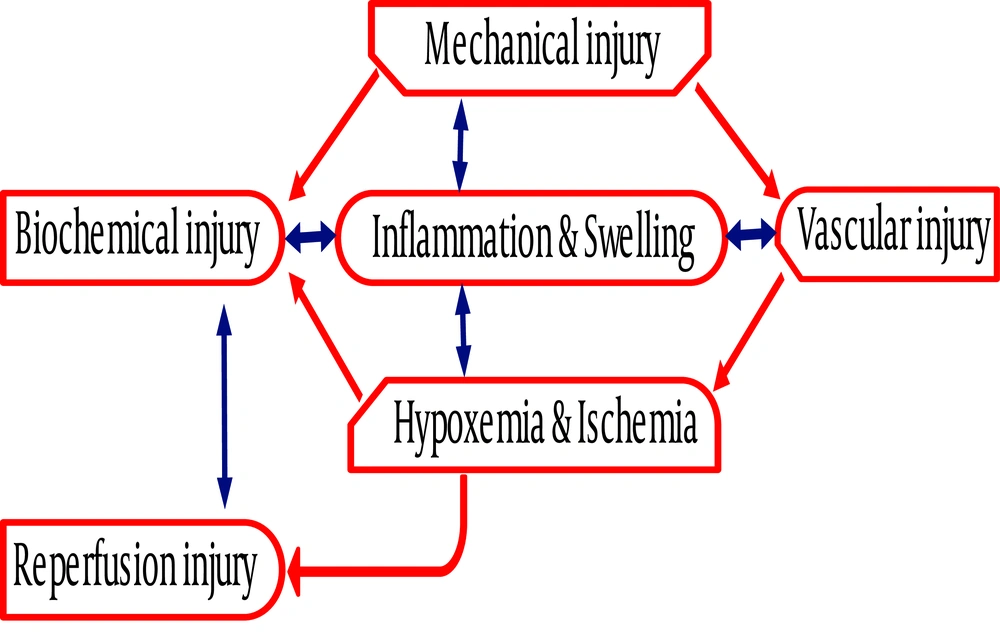
Following spinal cord injury, vascular disruption and ischemia ensue with a pathological cascade: electrolyte imbalance, release of lipid peroxide and generation of free radicals. These changes induce cellular membrane damage and trigger inflammatory reactions (Figure 1). Swelling and edema secondary to released inflammatory substances jeopardize regional blood flow leading to apoptosis and necrosis (1, 2). Treatment of spinal cord injury is started immediately to prevent further damage; restraining the spinal cord, removing external pressure, controlling inflammation and reducing edema are standard therapeutic strategies after trauma (1, 3). The non-surgical therapeutic measures include hypothermia, non-steroidal anti-inflammatory agents (NSAIDs), progesterone, glucosteroid, minocycline, osmotic diuresis, etc. (8). However, the level of injury, severity of initial damage and therapeutic time window are critical to predict the outcome of the patient (1, 8).
In animal studies, lidocaine infusion after brain injury reduces level of interleukin-6 and phospholipase-A2. Lidocaine infusion improved early electrophysiological recovery and reduced the size of the cortical infarct at 24 hours (9). N-acetyl-cysteine (NAC) is known as an antidote to acetaminophen overdose, but it has other multiple therapeutic effects and contradictory evidence. For example after brain injury in animal model, N-Acetyl-cysteine administration decreased inflammatory response and brain edema, ameliorated blood brain barrier (BBB) permeability, and prevented apoptotic cell death (10). Studies have shown the ability of the opiate receptor antagonist (naloxone) to improve recovery after experimental SCI. Significant benefit was observed in individuals with both neurologically complete (i.e. pelagic) and incomplete (i.e. paretic) injuries (7).
In preclinical and clinical studies, erythropoietin, asialo-EPO and carbamylated-EPO administration has markedly improved functional outcome after central nervous system or spinal cord injury (4-6). Erythropoietin induces a broad range of cellular responses in the nervous system that could protect and accelerate the healing process (Box 1). Erythropoietin has neuro-protective effects in in-vitro models of trauma, hypoxia, and hypoglycemia. In cultured neurons, erythropoietin prevents neuronal apoptosis and attenuates necrotic cell death. Erythropoietin also prevents excitotoxicity in neuronal cultures (11). Erythropoietin increases the activities of cytosolic antioxidant enzymes such as glutathione peroxidase and superoxide dismutase and inhibits lipid peroxidation; therefore, erythropoietin protects ischemic cells from oxidative damage (4, 5).
| Erythropoietin Neuroprotection Mechanisms |
|---|
| Anti-oxidative |
| Anti-inflammatory |
| Anti-apoptosis |
| Anti-glutamate |
| Maintenance of the blood brain barrier |
| Neurogenesis |
| Angiogenesis |
The others tissue-protective mechanisms of erythropoietin are its abilities to stimulate vascular endothelial growth factor secretion, increase angiogenesis and protect vascular integrity. It preserves blood-brain barrier integrity after injury by restoring expression of tight junction proteins (6, 12). In a in vitro, erythropoietin stimulates endothelial cells in vascular sites to reform the capillary tubes (5, 6). During oxidative stress and ischemic injury, erythropoietin displays direct antiapoptotic activity in cerebral endothelial cells. In experimental cerebral hemorrhage, endothelial nitric oxide synthase activity is induced by erythropoietin and it has been shown to contribute to the improvement of outcome (11).
In neural stem cell cultures, erythropoietin triggers neuroblastoma cells to differentiate into different neuronal cells (11). In animals models, erythropoietin increases proliferation of oligodendrocyte (8). Lack of erythropoietin receptor in mice during embryogenesis exhibits a reduction in the number of neural progenitor cells and more apoptosis in the nervous system (12). Moreover, erythropoietin has neurotrophic effects such as stimulation of axonal regrowth, dendritic sprouting, and electrical activity. All these mechanisms could explain the neurogenerative and recovery effects of erythropoietin after spinal injury. In addition, erythropoietin regulates intracellular calcium and neurotransmitter synthesis and release. However, it has not been clearly shown that erythropoietin makes new synapses (12-14).
Erythropoietin reduces inflammation by decreasing inflammatory cytokines, by attenuating reactivation astrocytosis and microglia activation and by inhibiting immune cells recruitment into the injured area (5, 13). Although several in-vivo studies have shown an anti-inflammatory effect of erythropoietin, the affirmation of its in-vitro effect is controversial (4, 15). In a clinical study, in patients with malignant extradural cord compression, a single high dose of erythropoietin (Epoetin alpha 1500IU/ kg IV infusion) before radiotherapy added to dexamethasone reduced the side effects of radiotherapy on spinal neurons without any major side effects (6). It has been reported that erythropoietin accelerates the recovery of visual loss after surgery (14).
On the other hand, long-term erythropoietin therapy in adults has serious complications (hypertension, clotting, seizures, polycythemia, and death). Fortunately, due to short-term use of erythropoietin (3 days in our patient), and new non-erythropoietic derivatives (like asialo-EPO and carbamylated-EPO) the chance of those complications is rare (5, 6, 13).