1. Background
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes. After 12 years of having diabetes, 30% - 50% of patients are affected by DPN. Painful diabetic peripheral neuropathy (PDPN) severely impairs quality of life and is the most distressing of all diabetic complications (1, 2).
The International Association for the Study of Pain defined peripheral neuropathic pain in diabetics as, “arising pain as a direct consequence of abnormalities in peripheral somatosensory system in diabetic patients.” The diagnosis relies on patient descriptions of pain, which is distal, symmetrical, commonly worse at night, and often deep, aching, sharp, and burning. Hyperalgesia and allodynia are usually present upon examination (3).
Unfortunately, a large number of pharmacological and non-pharmacological management techniques have had disappointing results. In some cases, patients choose to discontinue treatment because of adverse effects. Pain is considered to be unsuccessfully managed when pain continues to escalate with the use of more than three medications or when increasing medication doses are needed (4). Thus, it is a high priority to find new treatment methods with fewer adverse effects to manage DPN, and more specifically, PDPN.
Transcutaneous electrical nerve stimulation (TENS) and lumber radiofrequency sympathectomy are two therapeutic modalities that have had positive effects on PDPN. Transcutaneous electrical nerve stimulation (TENS) is a non-pharmacological, noninvasive treatment that has been used to treat a variety of painful conditions. The TENS technique reduces pain through peripheral and central mechanisms. This modality involves nerve stimulation by applying electrical current to the distribution of nerve fibers via skin surface electrodes. It triggers endogenous opioid release, modifies electrical transmission, and dilates blood vessels, all of which lead to a reduction in neuropathic pain (1, 4-6). Lumber sympathectomy is used to treat various neuropathic and ischemic conditions (7). Sympathectomy with radiofrequency (RF) is a minimally invasive procedure with a low incidence of adverse effects that can alleviate pain associated with peripheral neuropathies. This therapy can be delivered with two types thermal energy, continuous RF (CRF) and pulsed RF (PRF). In CRF a high frequency electric current (100,000 -500,000 Hz) is used to produce tissue temperatures of 45°C or more, which causes neuroablative thermocoagulation. Pulsed radiofrequency (PRF) is an alternative to CRF in which tissue temperatures remain below 45°C and tissue injury does not occur (8-11). The analgesic effects of PRF are thought to stem from rapid electric field changes and brief heat bursts that produce tissue temperatures that cause destructive heat lesions, but do not have an ablative effect. Therefore, PRF is safer and generally preferred over CRF because it can provide long lasting effects and is not associated with many of the adverse effects of CRF (e.g. lasting motor deficits, neuritis, and deafentation pain). It is possible to repeat this procedure (12-16). Decreasing sympathetic tone with lumbar sympathectomy causes vasodilation and tissue oxygenation. This has a direct neurolytic action on nociceptive fibers, interrupting sympathetic nociceptive coupling and allowing for pain relief (17).
2. Objectives
The aim of this study was to compare the efficacy of TENS and PRF lumbar sympathectomy in treating pain associated with PDPN.
3. Patients and Methods
This clinical trial was conducted at the Guilan Pain Clinic and Poorsina Hospital, both in Rasht, Iran. All participants provided informed consent, the study was approved by the institutional ethics committee of Guilan University of Medical Science, and the study was registered in the Iranian registry of clinical trials (201212166186N1). Subjects were enrolled between February 2013 and February 2015. Subjects were included if they had type I or type II diabetes mellitus, suffered from PDPN (diagnosed by a neurologist) that was resistant to conservative treatment for at least 6 months, had an Hba1c < 8%, a normal creatinine level, normal blood cell counts, and had a pain intensity of at least 4 on the 10-point Numerical Rating Scale (NRS). Subjects were excluded from study participation if they had an implanted pacemaker or heart defibrillator, implanted brain stimulator, history of alcohol abuse, malignancy, coagulopathy, anti-coagulant drug use, infection or irritation at the probe or electrode sites, or certain anatomical anomalies (1). Patients were instructed to continue conventional therapy (e.g. diet treatments, hypoglycemic agents), discontinue all analgesics, and take 300 - 600 mg pregabalin each day for the duration of the study (2).
Blinding patients to treatment assignments was not possible because the two procedures examined are so different. However, evaluating physicians were not aware of subject treatment assignments. Subjects in the PRF group were prepped in the operating room in a prone position in the usual sterile fashion. The procedure was performed at the L4-L5 level and on the affected side. In some cases, the procedure was performed bilaterally. The C-arm was first positioned using the oblique view at a 15-20 degree angle until the vertebral body covered the transverse process. The transverse process was aligned with the lateral edge of the vertebral body using the tunnel vision technique. Then the C-arm was laterally rotated to be at the pedicle level of the anterior-posterior (AP) view. An introducer (G16) was used for the procedure. A blunt, curved-tip needle (G22-150 mm) was placed through the introducer instead of the stylet.
After confirming the needle tip position in the oblique, AP, and lateral views, 2 mL of a contrast agent (omnipaque 320) was injected with no resistance and 2 mL of lidocaine 1% and 40 mg of triamcinolone were administrated under fluoroscope guidance. If an NRS reduction of at least 50% was reported within 6 hours, the patient underwent PRF lumbar sympathectomy using nearly the same technique described above for diagnostic nerve blockade. For this procedure, a 15 cm long, 22-gauge curved, blunt RF electrode with a 10 mm active tip (DIROS Technology) was used. After confirming needle position, the tip stylet was removed and the RF probe was placed.
Correct needle position was verified and motor nerve injury was avoided by using a 50 Hz sensory stimulus above a 0.4 V threshold (sensory test), followed by a 2 Hz stimulus above a 0.8 V threshold (motor test). After assuring correct needle placement in both the AP and lateral views, 2 mL of bupivacaine 0.5% was injected and the RF generator was activated, which pulsed RF energy at each level for three 180 second cycles at a temperature of 45°C. After the procedure, patients were monitored for 45 - 60 minutes. Following a normal neurological exam, patients were discharged. Clinical signs of regional sympathetic denervation were a warm, dry extremity and segment pain reduction. Skin temperature was recorded to test sympathetic blockade. Standard electrodes were placed on the dorsal and plantar surfaces of the foot and a third grounding electrode was placed remotely. A positive change of at least 2°C indicated a successful block (18-21).
In the TENS group one electrode was placed on the upper shin and one electrode was placed above the ankle.
Patients received TENS (80 Hz, 50 Amp, 0.2 ms square pulses, 2 to 3 times sensory threshold) for 20 minutes from a TENS stimulator (E3 model, Omron, [Omron location]). Ten TENS sessions were performed every other day. Pain evaluations were based on the NRS, which was measured four times before the procedure and 1 week, 1 month, and 3 months after the procedure. The NRS approach was used because it may provide the best balance between sensitivity and ease of use (22, 23). One physician who was blinded to treatment assignment evaluated the participants at each visit. Patients were asked to report side effects experienced within the 3 month follow-up period. Medical attention and other means were available for all study patients. If subjects in the TENS group were resistant to treatment, PRF lumbar sympathectomy was offered. If subjects in the PRF group were resistant to treatment, CRF was suggested. However, this did not occur during the study period.
3.1. Statistical Analyses
All statistical analyses were performed using SPSS statistical software (version 16, SPSS, Inc., Chicago, IL). Unpaired t-tests and chi-square tests were used to compare categorical variables between groups. Mann-Whitney U-tests were used to compare changes from baseline in NRS during the 3 months follow-up period. Data are expressed as mean ± standard deviation. Statistical significance was defined as P < 0.05.
4. Results
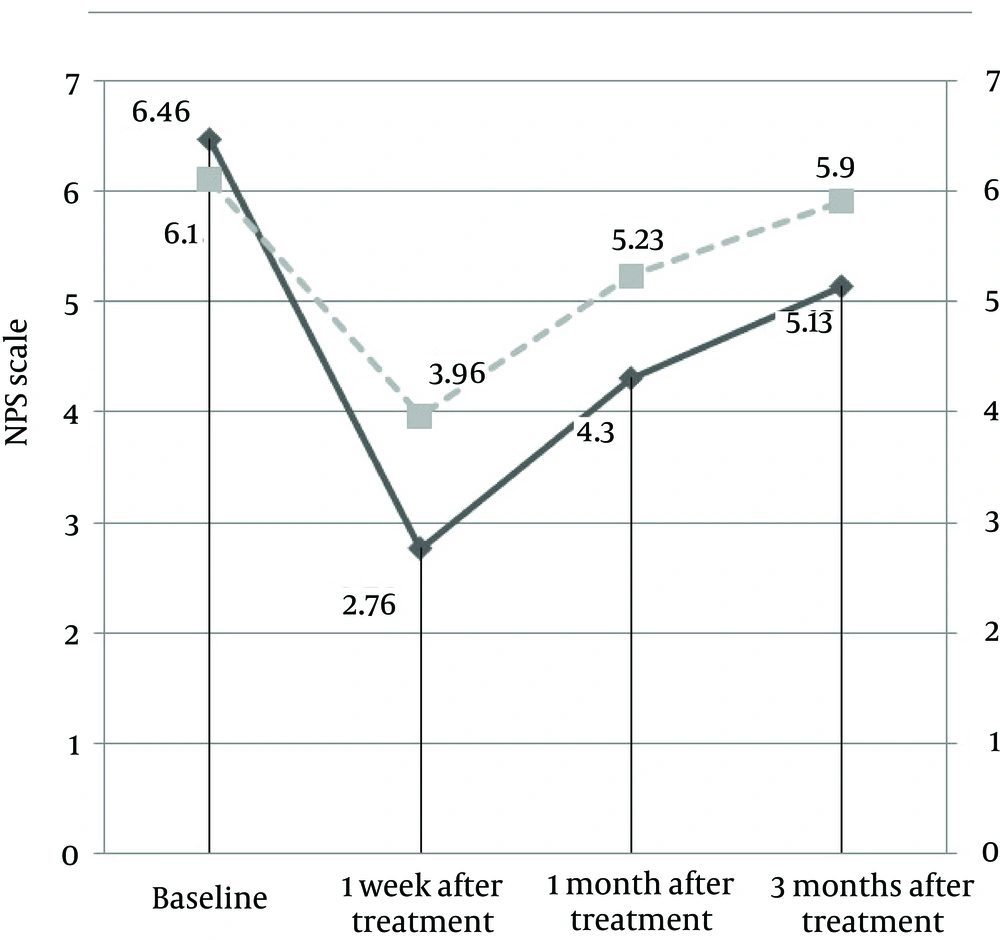
A total of 130 patients with PDPN were screened for inclusion/exclusion criteria during the above-mentioned study period. Of these, 55 patients did not meet all inclusion criteria and 10 patients did not wish to participate in the study for personal reasons. The remaining 65 patients were enrolled in this clinical trial and randomly divided into two groups (by triple blocks). In the PRF group, 3 subjects (2 subjects at the first follow-up visit, 1 subject at the second follow-up visit) chose to withdraw from the study for personal reasons. In the TENS group, 2 subjects chose not to complete the survey and 5 subjects were lost to follow-up and did not complete the survey. Therefore, 30 subjects were ultimately included in each study group for a total of 60 subjects (31 male [51.7%], 29 female [48.3%]). There was no significant difference between two groups in any baseline characteristic (Table 1) and mean participant age was 56.7 ± 6.37 years. An increase over baseline detected temperature was observed in all PRF subjects. Additionally, the NRS was significantly lower at follow-up visits than at baseline in both groups (P < 0.0001, Table 2). In the PRF group, NRS decreased from 6.46 at baseline to 2.76, 4.30, and 5.13 at the 1 week, 1 month, and 3 month follow-up visits, respectively (P < 0.0001). In the TENS group, NRS decreased from 6.10 to 3.96, 5.23, and 5.90 at the 1 week, 1 month, and 3 month follow-up visits, respectively (P < 0.0001). The PRF group had a marked reduction in NRS 1 week after treatment, but this decrease did not persist throughout the entire follow-up period. However, the NRS did not return to baseline levels. In the TENS group, a marked reduction in NRS was observed 1 week after treatment, but the NRS steadily increased to nearly baseline levels 3 months after treatment (Figure 1).
| Groups | NRS Score | P |
|---|---|---|
| 1 week after treatment | < 0.0001 | |
| PRF | 3.70 ± 0.59 | |
| TENS | 2.13 ± 0.89 | |
| 1 month after treatment | < 0.0001 | |
| PRF | 2.16 ± 0.74 | |
| TENS | 0.86 ± 0.68 | |
| 3 months after treatment | < 0.0001 | |
| PRF | 1.33 ± 0.88 | |
| TENS | 0.20 ± 0.40 |
5. Discussion
Painful neuropathy is the most disabling of all diabetic complications and results from peripheral nerve damage. The peripheral nervous system is affected in both type1 diabetes and type II diabetes. Pathophysiology of PDPN remains unknown, but several trials have shown that tight glycemic control reduces the occurrence and progression of diabetes related complications. Unfortunately, this approach alone cannot completely eliminate complications. Despite wide and varied treatment options, optimal analgesia is often not achieved (24-26). Several studies have shown that PRF lumbar sympathectomy and TENS can successfully treat neuropathic pain. In 2008, Bruton et al. (27) reported a PDPN case that did not respond to conventional treatments. Lumbar sympathectomy was performed on this patient using a fluoroscopy technique at the L3-4 level. Following the procedure, the NRS decreased from 7 to 3. In 2010, Jin et al. (4) reported that TENS therapy is safe and effective for treating symptomatic DPN. In 2004, Forts et al. (28) found that low frequency TENS is a non-pharmacological treatment option for primary or advanced DPN.
Gossrau et al. (1) performed a randomized, placebo-controlled study and showed that TENS is not superior to placebo in managing DPN. They suggested that electrode location and disease stage may have variable effects on therapeutic outcomes (1). In 1998, Kumar et al. (29) showed that TENS therapy had provided positive results in patients with painful DNP who failed to respond to amitriptyline therapy.
To the best our knowledge, the effects of the two therapeutic methods studied here have not been previously compared in patients with PDPN. Our study compared the efficacy of these two modalities in improving PDPN symptoms. We hope our study findings will result in further similar studies. The therapeutic methods studied here were well tolerated were not associated with any serious adverse effects. However, skin irritation was reported in a few TENS group subjects. The results of this study support previous findings by others and indicate that both PRF sympathectomy and TENS therapy can relieve pain associated with DNP. Additionally, our results showed that PRF sympathectomy had a superior effect. Unfortunately, the long-term effects of TENS and PRF sympathectomy remain controversial. Large multi-center randomized, controlled trials are needed to evaluate the long-term efficacy of these procedures.
5.1. Limitations
Our study had several limitations. First, our study included a relatively small number of subjects. We do not know if treatment efficacy will continue over the long-term and we cannot be sure enough about the long-term safety of the studied procedures. Studies with a follow-up period longer than 3 months are needed. Third, different TENS stimulation parameters and lumbar sympathectomy methods may have influenced our data and resulted in different findings. Studies examining the effects of different stimulation and procedural styles are needed.
In conclusion, both PRF sympathectomy and TENS can reduce lower extremity pain in patients with PDPN. However, PRF sympathectomy seems to be more effective than TENS.