1. Introduction
Eventration and central tendon defect of diaphragm give way to herniate abdominal contents into the thorax in congenital diaphragmatic hernia (CDH). Its incidence is approximately 1 in 3000 live births with a reported mortality of 40% - 62% depending on the associated major malformation (1). As a consequence of underdevelopment of the lung parenchyma, due to herniated abdominal contents in thorax, abnormal pulmonary vasculature growth may occur resulting in increased pulmonary vascular resistance and pulmonary hypertension (PAH). A recent study in collaboration with the CDH registry reported a 41% prevalence of bronchopulmonary dysplasia (BPD) in survivors of CDH, about one-third of the cases are associated with cardio-vascular malformations, and lesser proportions have skeletal, neural, genitourinary, gastrointestinal or other defects (2). BPD associated with pulmonary hypertension carries a higher risk of pulmonary morbidity. It is a standard practice to ventilate such neonates electively in postoperative period to recruit the alveoli and maintain oxygenation (3). Perioperative sympathetic stimulation due to pain, response to endotracheal tube, hypoxia, hypercarbia and hypervolemia will aggravate PAH and adversely affect the postoperative outcomes.
Dexmedetomidine has predictable sympatholytic, analgesic, hypnotic and anxiolytic effects without respiratory depression and its pharmacologic effect is mediated through post-synaptic α-2 adrenergic receptors present in medullary vasomotor center, locus ceruleus and dorsal horn of spinal cord (4). It is safe even in doses high enough to cause unresponsiveness (5). In adult intensive care units (ICUs), it is helpful to reduce traditional sedative/analgesic use while still allowing for a calm, comfortable and cooperative state (6). In adults with pulmonary hypertension undergoing mitral valve replacement, dexmedetomidine effectively attenuated the increase in mean arterial pressure (MAP), pulmonary arterial pressure (PAP) and pulmonary capillary wedge pressure (PCWP) (7, 8). Effects of dexmedetomidine 0.62 μg/kg loading dose followed by an infusion of 0.5 μg/kg/hour on PAP and pulmonary vascular resistance (PVR) were evaluated in a cohort of 22 pediatric patients following the congenital heart disease surgery using echocardiographic analysis of tricuspid regurgitant velocity showed reduction in pulmonary artery systolic pressure as well as pulmonary artery systolic pressure to systemic systolic pressure ratio (9). Additionally, anecdotal success is also reported with the use of dexmedetomidine for sedation in high risk patients with pulmonary hypertension (10-12). Based on its efficacy in adults, it is explored as an alternative or adjunct to benzodiazepines and opioids in the pediatric intensive care setting (13). Neonatal experience with dexmedetomidine is predominately in the form of case series and small reports, mainly focusing on its short term or procedural use. In neonatal congenital diaphragmatic hernia associated with pulmonary artery hypertension, perioperative use of dexmedetomidine is expected to result in stable hemodynamics, smooth elective mechanical ventilation and decrease in PAP. Thus this study of extended dexmedetomidine infusion was planned in neonatal corrective surgery of CDH with PAH in an attempt to assess its effect on perioperative hemodynamics and oxygen saturation.
2. Case Presentation
2.1. Patients
2.1.1. Case 1
A 2 kg premature male neonate, born via caesarean section, developed respiratory distress at birth (APGAR score of 6 at 1 and 7 at 5 minutes), was shifted to neonatal intensive-care-unit (NICU), where with oxygen support, oxygen saturation was maintained (SpO2) > 92%. Examination revealed scaphoid abdomen, barrel chest, reduced air entry on the left side of chest with shift of apical impulse to the right side. Further investigations confirmed the diagnosis of CDH associated with PAH, as shown by echocardiography, and small gut obstruction for which emergency corrective surgery was planned.
2.1.2. Case 2
A 2.5 kg premature female neonate, born via caesarean section, developed respiratory distress at birth (APGAR score of 7 at both 1 and 5 minutes). On detailed examination, she had radial hypoplasia and echocardiography revealed PAH, and emergency corrective surgery of CDH was planned.
2.1.3. Case 3
A 1.8 kg premature male neonate, born via caesarean section for meconium stained liquor, developed respiratory distress (APGAR score of 6, both at 1 and 5 minutes), with SpO2 of 89% even with oxygen supplementation, neither associated with cyanosis nor symptoms of shock. Chest radioimaging and echocardiography confirmed the diagnosis of CDH with association of severe pulmonary hypoplasia and PAH. Emergency corrective surgery was planned.
2.2. Methods
After the provision of written informed consent from the parents, the patients were shifted to operating room. Duration of the study was 20 minutes prior to induction of anesthesia up to postoperative 48th hour. Standard monitors which recorded base line and subsequent parameters were attached. Neonatal targeted temperature was 36.28°C to 36.58°C. Just before induction of anesthesia, each patient received IV glycopyrrolate 10 µg/kg followed by dexmedetomidine bolus 1 µg/kg over 20 minutes. Anesthesia was induced with titrated IV propofol (2 - 3 mg/kg) and after adequate ventilation with 100% oxygen, atracurium 0.5 mg/kg was administered and trachea was intubated at adequate muscle relaxation. Mechanical ventilation was initiated targeting minute ventilation to maintain normocarbia and FiO2 to maintain SpO2 above 90% (pressure control mode and positive end-expiratory pressure of 5 cm H2O), after securing endotracheal tube and confirming bilateral air entry. Anesthesia was maintained with oxygen, compressed air, isoflurane and titrated dexmedetomidine infusion (0.3 - 0.7 µg/kg/hour). IV fluid was given as per standard protocol and before skin incision IV fentanyl 2 μg/kg was administered. IV fentanyl and atracurium were repeated as per requirement of analgesia and muscle relaxation during the surgery. At the end of the surgery, rectal paracetamol suppository 40 mg/kg was placed and fresh isoflurane and compressed air flow were terminated. Patients were shifted with tracheal tube in-situ to NICU and put on elective mechanical ventilation (SIMV/PC mode) targeting SpO2 > 92% and end-tidal carbon-dioxide (EtCO2) of 35 - 45 mmHg. Dexmedetomidine infusion was continued at the same rate for the next 48 hours and was tapered to 0.3 µg/kg/hour over the last four hours. Rectal paracetamol suppository was repeated at intervals of six hours and IV fentanyl 1µg/kg bolus was used as rescue analgesic. Extubation was planned to fulfill the extubation criteria.
2.3. Observations
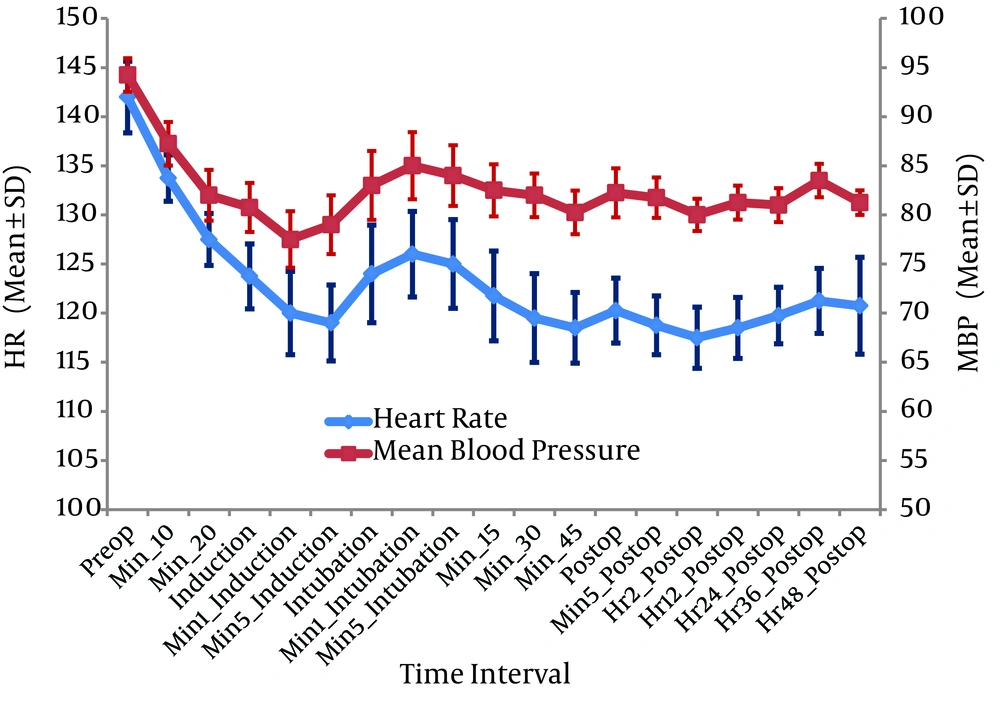
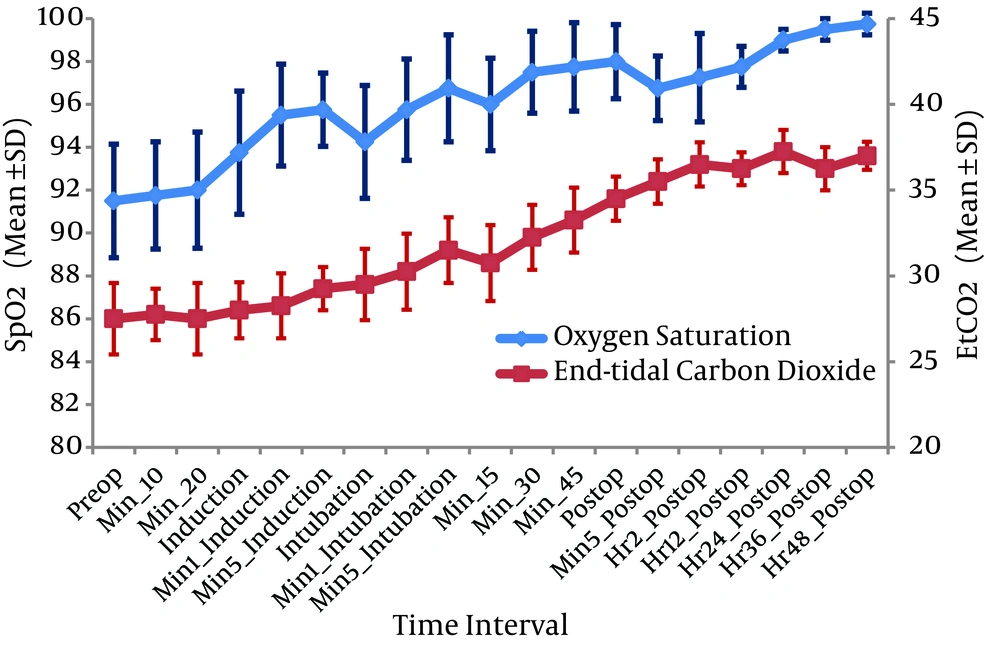
During the study heart rate (HR) and mean blood pressure (MBP) remained virtually unchanged with adequate minute ventilation in all the three neonates (Figure 1). They experienced few episodes of bradycardia and hypotension at the upper end of dexmedetomidine infusion (above 0.5 µg/kg/hour), and since the dose of infusion was titrated on the lower side (0.3 - 0.5 µg/kg/hour), it reversed. None of the patients required any drugs or resuscitative manoeuvres for the adverse effects. Postoperative SpO2 was well above 96% with inspired oxygen fraction (FiO2) of 0.5 in patients 1 and 2, and 90 - 94% with FiO2 of one in patient three; with a gradual improving trend of both SpO2 and EtCO2 (Figure 2). They were pain free based on the neonatal infant pain scale (average NIPS was between 0 - 4) and on the objective assessment with Ramsay sedation score (RSS of 3 - 4). Single rescue analgesic was required in patient three at 40th post-operative hour. There was no incidence of post-operative shivering in any of them.
After one hour of terminating the dexmedetomidine infusion, patients were opening eyes on external stimulus (RSS was around 3) and they were successfully extubated after fulfilling extubation criteria. No withdrawal responses such as agitation, tremors or decreased sleep, were observed on stopping the infusion and hemodynamics was stable. The subjects were shifted from NICU to ward on the 14th post-operative day when general conditions improved and discharged from hospital on the 30th post-operative day. Upon regular follow up at six months, developmental milestones were normal in all the three patients.
3. Discussion
In the present study, extended perioperative IV dexmedetomidine infusion in neonates was well tolerated. Reiter et al. in their retrospective study of more than 24 hours dexmedetomidine infusion in critically ill infants and children concluded that it was well tolerated (14).
The current study used perioperative dexmedetomidine infusion in congenital diaphragmatic hernia with pulmonary hypertension in the emergency corrective surgery and stable hemodynamics was observed. dexmedetomidine is extensively used in adults and pediatrics for sedation and as an adjuvant to anesthesia (15, 16). Tokuhira et al. compared dexmedetomidine to standard analgesic/sedative combinations for sedation after Fontan surgery and concluded that dexmedetomidine improves cardiac function and decreases elevated pulmonary vascular resistance (17). Chrysostomou et al. used dexmedetomidine in children after cardiac and thoracic surgeries and concluded that dexmedetomidine has cardio stable property (18). Adverse cardiovascular effects of dexmedetomidine are limited and include occasional episodes of bradycardia and hypotension that are mainly with rapid administration of boluses (7, 19-21).
The current study used dexmedetomidine infusion for 48 hours post operatively aiming to provide smooth elective mechanical ventilation. Shehabi et al. in their study on critically ill ventilated patients concluded that hypnotic effect of dexmedetomidine mimics normal sleep with an advantage to tolerate tracheal reflex and stress of mechanical ventilation (22). Chrysostomou et al. reported its increased elimination half-life (7.6 vs 3.2 hours) in preterm neonates (23). But the current study patients opened eyes on external stimulus after one hour of terminating the infusion and were successfully extubated after fulfilling extubation criteria. It is apparent that physical dependency and withdrawal might occur following the prolonged administration of dexmedetomidine (24). But several recent prospective studies targeted toward prolonged infusion of dexmedetomidine when treating critically ill patients in the ICU, with the benefits clearly outweighing the little risks involved (25). No withdrawal responses such as agitation, tremors or decreased sleep were observed in the patients, most probably because the infusion gradually was tapered over the last four hours before its termination.
Postoperative oxygen saturation was above 96% with FiO2 of 0.5 in cases 1 and 2, and 90% - 94% with FiO2 of one in case three. Low birth weight associated with PAH and pulmonary hypoplasia might have attributed to increased FiO2 requirement in case three. With the preliminary report, authors were unable to conclude whether improved oxygen saturation was due to perioperative stable hemodynamics and smooth mechanical ventilation or the property of the drug to reduce pulmonary vascular resistance. Safe discharge of patients from hospital with normal developmental mile stones at six month follow up was observed.
Neonates experience more pain in response to noxious stimulus, rather than less as believed previously, due to combination of widened receptive fields, lower sensory discrimination and immature nervous system with reduced inhibitory pathways (26). It was observed that neonates were pain free (average NIPS 0 - 4) with good sedation (average RSS was around 3 - 4). Single dose of rescue analgesic was required in patient three at the 40th hour in the post-operative period (NIPS 6). Reiter et al. in their retrospective study concluded that dexmedetomidine possesses analgesic and sedative sparing properties (14).
Based on the results of the present study, it can be concluded that intravenous dexmedetomidine infusion, when used as a perioperative pharmacologic adjunct, in the emergency corrective surgery of congenital diaphragmatic hernia associated with pulmonary artery hypertension, results in stable hemodynamics with satisfactory oxygen saturation.
Based on the preliminary report, authors were unable to conclude regarding its safety in neonates. Neither pulmonary arterial pressure nor cardiac output were monitored during the study, consequently, it remains unclear whether the improved oxygen saturation was due to perioperative stable hemodynamics and smooth mechanical ventilation, or the property of the drug to reduce pulmonary vascular resistance. Further large multicentric studies are warranted for definite conclusion on these issues.