1. Background
One of the main concerns that patients deal with surgery is pain after. Nowadays, treating postoperative pain is highly regarded. Pain, nausea vomiting and drowsiness are the most common causes of delayed recovery (1).
Hysterectomy is one of the most common procedures and, currently, holds the second place after caesarean section (2). The pain following abdominal hysterectomy results in a delay in the intensive therapy recovery of patients after surgery, longer hospitalization period, chronic pain, increased probability of venous thrombosis and dissatisfaction of patients. One of the methods for controlling postoperative pain is preemptive analgesia (1, 3). Preemptive analgesia hypothesis states that, if analgesia started before the initial stimulation of the surgery, it would be more effective than after an acute stimulation. Several studies have described the efficacy of preemptive pain control strategies (4-7). Prescribing analgesics before the onset of pain, instead of when the patient reports pain, provides a chance for the analgesic drug to reach the appropriate serum level and, therefore, the pain can be managed more rapidly, effectively, and uninterruptedly (8-11). Anticonvulsant drugs are mostly used in controlling chronic neuropathic pains. Antineuropathic medication, such as gabapentin and pregabalin, is believed to play a role in postoperative pain management (4, 12, 13). Gabapentin, a well tolerable anti-epileptic drug with limited adverse effects, has analgesic, anticonvulsant and anxiolytic effects. It is structurally considered an analog to aminobutyric acid. Its mechanism of producing analgesia is unknown. This drug has a structure similar to γ-aminobutyric acid (GABA); however, it does not interact with its receptors. It is believed that the drug increases the general concentration of GABA in the brain, through an unknown mechanism. Tramadol is an agonist of µ receptors and noradrenergic and serotonergic systems, and releases serotonin (3, 14). Considering the importance of the issue, it is demanded to investigate for postoperative pain control modalities, with superior effectiveness and less side-effects, for this type of surgery, with a high prevalence. As opioids are known to have adverse effects, especially concerning dependency, we tested the hypothesis that if gabapentin, as a non-opioid agent, could reduce postoperative pain, compared to tramadol. Then, our promising results would lead to reducing opioid requirements and their related side-effects, such as respiratory depression and preventing from opium tolerance.
2. Objectives
In this study, the effects of preoperative administration of these drugs, in the control of postoperative pain and analgesic requirements, have been investigated.
3. Patients and Methods
This randomized double-blind clinical trial took place in Alzahra Academic Hospital, Guilan University of Medical Sciences, Rasht, Iran, from September 2014 to May 2015. The trial protocol was approved by the Research Ethical Committee of Guilan University of Medical Sciences, Rasht, Iran and also registered in Iranian Registry of Clinical Trial; IRCT number: 201201108677N1. All participants provided their informed consent.
The study included patients aged 35 - 55 years old, with American Society of Anesthesiology class I‒II, body mass index (BMI) of 20 - 25 kg/m2, who underwent elective abdominal hysterectomy, in one of the teaching hospitals affiliated with Guilan University of Medical Sciences, Rasht, Iran. Patients were excluded from the study if there was a history of cardiac disease, pulmonary disease, renal disease, systemic hypertension, high intracranial pressure, hepatic diseases, psychological disease, asthma, chronic pain syndromes and seizure. Furthermore, the patients, who received routine preoperative analgesia, had a history of drug abuse, or were allergic to gabapentin and tramadol, did not enter the study, either. Patients were recruited one day before the surgery and were interviewed for a medical and drug history and underwent a physical exam. Venous blood was obtained for baseline biochemical serum measurements (cell blood count, liver function tests, serum creatinine). In addition, they had a 12 lead ECG. If the patients did not have any history of psychological diseases or consume any illegal drugs, they could be enrolled in the study.
The study was double-blinded, therefore the patients and the evaluators were unaware of the study groups that each patient belonged to, however the administrator was aware from the type of drug. All patients were fasting for 8 hours before surgery. They were allocated to one of the gabapentin, tramadol, or placebo groups, using randomized fixed blocks (quadripartite blocks). Two hours before surgery, patients in the gabapentin, tramadol, and placebo groups, respectively, received Tab Gabapentin 300 mg (Alhavi Pharmaceutical Company, Tehran, Iran), Tab Tramadol 100 mg (Alhavi Pharmaceutical Company, Tehran, Iran) and placebo, administered with 50 ml of water. In the operating room, heart rate, blood pressure and arterial oxygen pressure were recorded, before establishing venous cannulation. Induction of anesthesia was established by administration of midazolam 0.07 mg/kg, fentanyl 2 µg/kg and propofol 2 mg/kg. For muscle relaxation and tracheal intubation, atracurium 0.5 mg/kg was given. Isoflurane, along with nitrous oxide and oxygen 50/50, were administered for maintenance of anesthesia. Patients then underwent abdominal hysterectomy. All the operations were performed by one surgeon. Duration of surgery, for each patient, was of approximately 2 hours. At the end of the surgery, neuromuscular blocking effects of atracurium was reversed, by administrating atropine 0.02 mg/kg and neostigmine 0.04 mg/kg, the tracheal tube was removed and patients were transferred to the post-anesthesia care unit. In the postoperative period, if patients complained of pain, they received diclofenac suppository 1 - 3 mg/kg, every 30 minutes. Pain score, nausea, vomiting, sedation, satisfaction rate and the number of doses of meperidine in 24 hours were recorded in the time intervals of 1 (T1), 4 (T2), 8 (T3), 12 (T4), 16 (T5), 20 (T6), 24 (T7) hours after the end of the operation. Should patients show a visual analog pain (VAS) score > 3, intravenous meperidine 0.25 mg/kg was administered. In case of nausea and vomiting, metoclopramide 10 mg IV was given. All data were gathered and analyzed by SPSS 21 software (SPSS Inc., Chicago, IL, USA).

4. Results
A total of 120 patients, aged 35 - 55 years old, were selected for the study and allocated to one of the three groups of gabapentin, tramadol and placebo. Chi-square test showed that the three groups were not significantly different, in terms of age (P = 0.47) and BMI (P = 0.7).
The BMI in the 3 studied groups were: tramadol group = 22.6 ± 1.58; gabapentin group = 23 ± 1.75; placebo group = 23.2 ± 1.57. The average of age, in the three studied groups was: tramadol group = 50.22 ± 12.54; gabapentin group = 48.12 ± 7.68; placebo group = 47.97 ± 5.8 years old.
General linear model and repeated measurement were applied to determine the trend of changes.
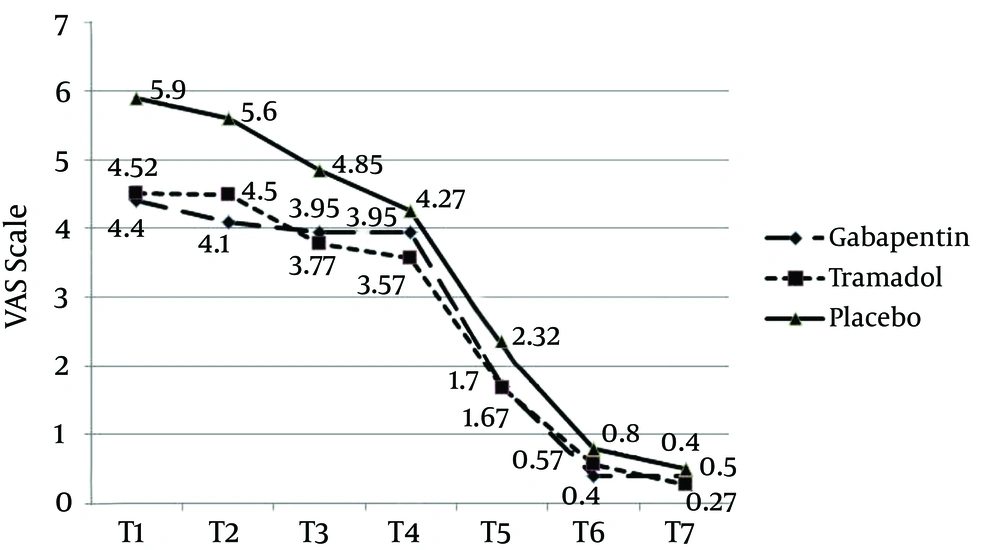
Patients in the placebo group had higher VAS, during the first hours (P = 0.0001), compared with the gabapentin and tramadol groups. Twelve hours after the surgery, all three groups showed downward trends of the pain VAS and at the 20th hour, almost all patients had no pain. The gabapentin and tramadol groups showed similar trends of changes in the pain VAS; however, in the first hours (up to 8 hours) analgesia rate was higher for the gabapentin group, compared to the tramadol group. From 8 to 11 hours onward, VAS was lower for the tramadol group (Figure 1).
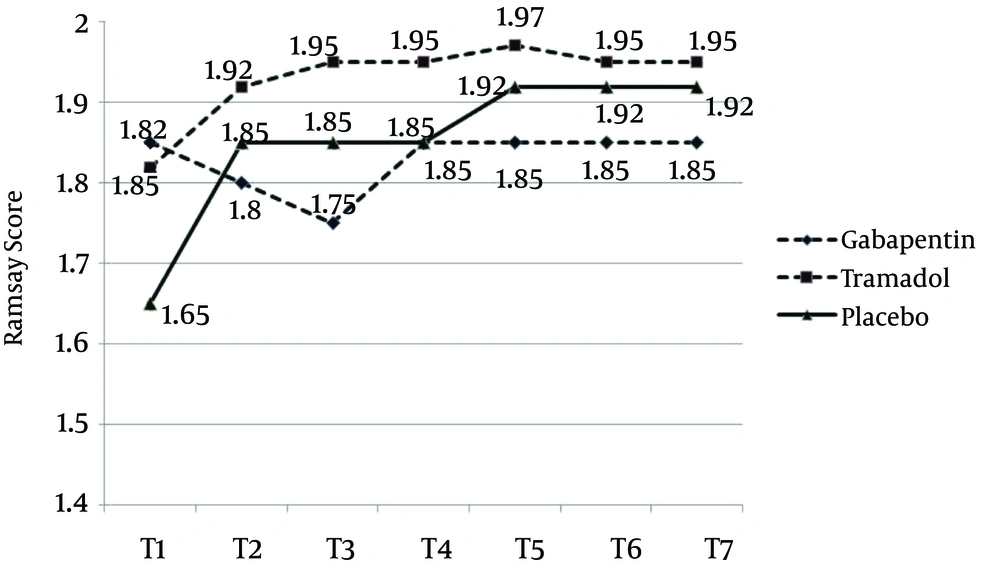
In the early hours, the Ramsay score was lower for the placebo group, compared with the other two groups (patients were more conscious) and the sedation level was higher in the tramadol group, compared with the two other groups. These differences, however, were not statistically significant (Figure 2).
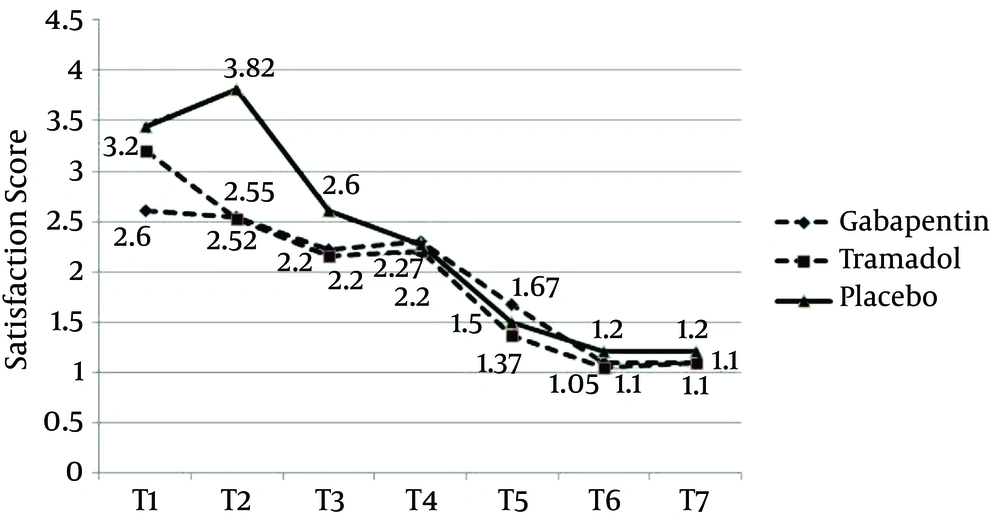
Satisfaction score trends showed that patients in the placebo group were significantly more dissatisfied in the early hours, especially at the 4th hour, compared with the two other groups (P = 0.0001). Changes in satisfaction score were similar in the gabapentin and tramadol groups, although satisfaction score in the early hours (the first 4 hours) was significantly higher for the gabapentin group, compared with the tramadol group (P = 0.0001) (Figure 3).
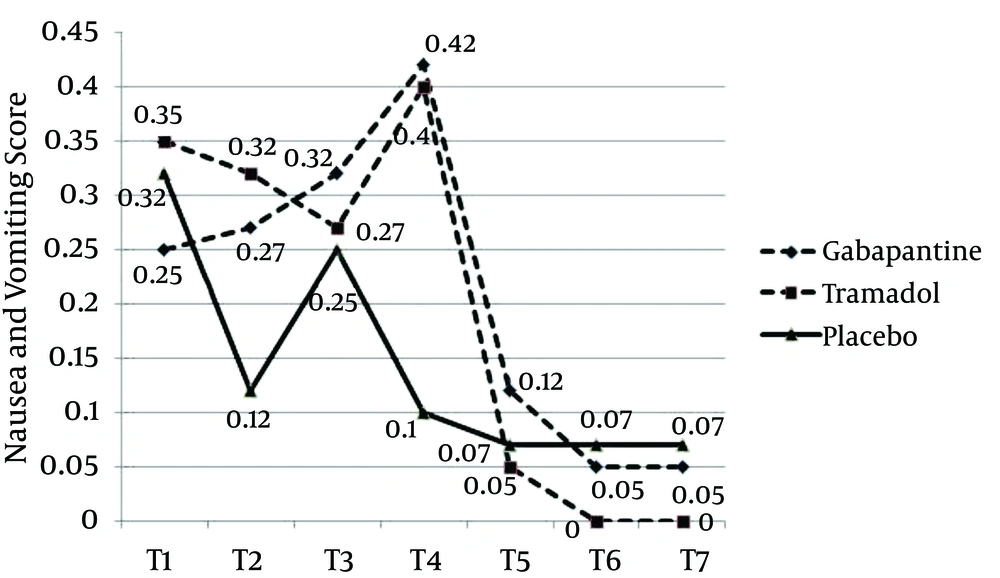
Nausea and vomiting score was lower for the placebo group, compared with the two other groups; however, this difference was not statistically significant (P = 0.29). Nausea and vomiting scores, for the gabapentin and tramadol groups, showed different trends, until 12 hours after the operation, and similar trends afterwards. For the gabapentin group, nausea score was lower in the first hour and increased during the next 12 hours. Contrarily, for the tramadol group, nausea score was higher at first, showing downward trend during the next 8 hours, and increased afterwards until the 12th hour. In both groups, nausea and vomiting scores decreased after the 12th hour, reaching even zero after 20 hours, for the tramadol group (Figure 4).
The average administered dosage of meperidine was determined using Fisher’s exact test. During the first 24 hours after the operation, average meperidine dosage was significantly higher for the placebo group [62.55 mg compared with 18.75 and 17.5 mg for the gabapentin and tramadol groups, respectively (P = 0.0001)]. Overall, 17 patients in the gabapentin group (34%) and 16 patients in the tramadol group (32%) received meperidine and Chi-square test determined that there is no statistically significant difference between the two groups, in this regard (P = 0.832).
5. Discussion
Our study revealed that gabapentin 300 mg, two hours before the surgery, was more effective in reducing pain and VAS score, in comparison to tramadol 100 mg and placebo. Even though pain reduction in gabapentin and tramadol group was the same, analgesia rate during the initial hours was higher in the gabapentin group. Ramsay score, in the placebo group, was lower (patients were more conscious) than the gabapentin and tramadol groups. Satisfaction rate in the placebo group was lower and the patients reported their dissatisfaction, especially in the first 4 hours. Satisfaction rates for the tramadol and gabapentin groups were almost the same, except the first four hours, when satisfaction score was higher in the gabapentin group. Nausea and vomiting rates in the placebo group were lower than in the other groups. Patients in the tramadol group had a high nausea and vomiting score, from the early hours, and felt dissatisfied. The amount of meperidine administered in the placebo group was significantly higher, while it was almost the same for the gabapentin and tramadol groups. The request for meperidine in the gabapentin group, however, was higher than the tramadol group.
Preventing and treating pain and complications, such as postoperative nausea and vomiting, are major concerns in the postoperative care and play an important role in facilitating the patient’s postoperative recovery and satisfaction. Postoperative pain is caused by surgical stimulation and neural factors, such as visceral tissue edema. Opioid analgesics, with their well-known complications, still form the basis for postoperative pain management (3, 15). Current therapeutic methods for pain include analgesic medications, with different mechanisms. Gabapentin was initially used as an anticonvulsant drug; however, recent studies have revealed its anti-hyperalgesic effects. Studies on animals showed pre-surgical treatment with gabapentin is more effective in reducing and controlling pain and allodynia, in comparison with treatment administered after the surgery. There is a possibility that gabapentin causes this effect, as a result of synaptic attachment to α2 and µ1 subunits of calcium voltage-dependent channels of dorsal horn neurons, in the spinal cord, which reduce the entry of calcium into the nerve terminals and, therefore, inhibit the release of neurotransmitters. However, there are conflicting results regarding the impact of gabapentin on pain and opioid use. Preemptive analgesia is one of the postoperative pain management methods (3, 15, 16). In fact, controlling pain, in patients that experience severe pain in the emergence period, is far more difficult compared to the patients who awake smoothly.
In a study by Turan et al. (17) the effect of gabapentin 1200 mg on pain and the amount of intravenous tramadol use, following hysterectomy, were investigated. It was observed that, in the gabapentin group, both parameters decreased in the first hours. In our study, a 300 mg dose of gabapentin was used, which was one fourth of the dosage in Turan’s case; however, patients experienced a satisfying level of analgesia after the surgery, while the rate of side-effects associated with high gabapentin dosage, including nausea, vomiting and sedation, was lower in our study, compared to Turan’s (17).
In a study by Durmus et al. (18), the effects of 1200 mg gabapentin, gabapentin 1200 mg with acetaminophen 20 mg/kg and placebo, 1 hour before hysterectomy, were evaluated. Pain intensity and morphine requirement, in both treated groups, decreased compared to the placebo group. However, there was no significant difference in pain score reduction between the gabapentin and gabapentin plus acetaminophen group and pain alteration trends were similar, as well. The authors, accordingly, suggested using a single dose of gabapentin before hysterectomy, in order to reduce postoperative pain.
Dierking et al. (19) studied the effect of 1200 mg gabapentin 1 hour before the surgery, in respect to placebo, in hysterectomy patients, showing considerable decrease in pain, nausea and vomiting in the gabapentin group. Nevertheless, in our study, nausea was lower in the placebo group, compared to the other two groups, and the highest nausea and vomiting rate, in the first hours, belonged to the tramadol group, probably because of opioid adverse effects of tramadol.
Rorarius et al. (20) gave gabapentin 1200 mg or oxazepam 15 mg (active placebo) 2.5 hours prior to induction of anesthesia to patients undergoing elective vaginal hysterectomy. Gabapentin reduced the need for additional postoperative patient-controlled analgesia (PCA) by 40%, during the first 20 postoperative hours. Especially during the first hour after the operation, postoperative pain scores, at rest, were significantly higher in the placebo group, compared with patients who had received gabapentin. These findings were consistent with our results; however, anxiety rate was lower in the active placebo group of their study, which is probably due to sedative effects of oxazepam.
In the study conducted by Fassoulaki et al. (21), the patients were allocated to two groups of gabapentin and placebo. The patients in the gabapentin group received gabapentin 600 mg every 6 hours, starting from 18 hours before surgery, until the 5th postoperative day. The pain score and morphine request rate were assessed for 2‒3 days and 1 month after the surgery. Gabapentin decreased morphine requirements for the first 48 h after the surgery. However, neither it altered the analgesic requirements beyond 48 h, nor had any effect on late or chronic pain.
In our study and most of the other studies that found a significant effect of gabapentin on postoperative pain, single dose of gabapentin was administered. This effect can be related to higher efficacy of a single dose of gabapentin, compared to multiple doses. Likewise, according to current evidence, 1 - 2 hours before surgery was the best time for the administration of the premedication in our study and other similar studies and was associated with the optimum results, probably due to the peak effect of the premedication drug.
Frouzanfard et al. (22) gave gabapentin 1200 mg to a group of hysterectomy candidates and placebo to the other group, 2 hours before the surgery. Pain and required morphine in the gabapentin group decreased significantly, similar to our study. However, in this study, high dose gabapentin caused nausea and vomiting, in the first hours. The recommended dosage for gabapentin, in respect to type of surgery, is between 300 to 3000 mg, in time intervals, according to different studies. The proper dose may be chosen based on type of surgery, intensity of inflammation, tissue damage and type of pain-somatic or visceral. On the other hand, increasing the dose to more than 1200 mg results in nausea, vomiting and dizziness, in patients. In our study, low dose gabapentin (300 mg) was administered and propofol was used for induction of anesthesia. Therefore, the patients experienced less nausea and vomiting in the early hours.
Our study showed that the trends of reductions in the pain score and satisfaction rates following abdominal hysterectomy were similar after administration of gabapentin 300 mg or tramadol 100 mg, 2 hours before the surgery. Complications, such as nausea, vomiting and sedation, were more common after tramadol, which is expected, as it is an opioid analgesic. On the other hand, gabapentin is a non-opioid drug, which has been as effective as tramadol in controlling postoperative pain, without any of its complications. Therefore, gabapentin can be a proper alternative to opioids for premedication.