1. Background
Mechanical ventilation (MV) completely abolishes natural airway defense mechanisms. As a result, MV may damage the respiratory tract and have other negative impacts on patient’s respiratory system. Ventilator associated pneumonia (VAP) is the main concern in this scenario and is one of the major causes of morbidity in the intensive care unit (ICU) (1).
VAP is associated with increased hospital stay, longer MV duration and prescription of antibiotics, thereby raising healthcare costs and possibly resulting in higher mortality (2-4).
Moisturizing, heating and filtering gases inspired via the MV circuits help to reduce the adverse effects of MV. However, there is still no consensus on whether these measures improve patient prognosis, shorten MV duration, decrease airway secretion and lower the incidence of VAP and other complications (5, 6).
MV equipment able to moisturize, heat and filter gases entering the airways is available in the market. The benefits of such technology require better and continuous assessment, especially in the case of long-term MV. Various aspects such as moisturizing time, duration and mechanism must be considered. The gases inspired by mechanically ventilated patients can be heated and moisturized by active mechanisms, represented by heated humidifiers (HH), the first and most often used equipment or passive mechanisms, represented by heat and moisture exchangers (HME), which have been recently introduced for ICU setting and have been increasingly used for MV (7-10).
Some authors advocate the use of HME, but most clinical trials and systematic reviews have not detected any significant differences in the number of VAP events during the use of HME and HH (with and without meta-analysis of clinical trials) (11-15).
Some studies demonstrated that changing HME every 24 hours leads to lower daily cost per ventilated patient (12, 16, 17).
Additionally, some papers have stated safety of changing HME every 48 hours or even after longer ventilation periods, which should reduce costs even further. Because no investigations have evaluated cost-effectiveness of HME in terms of VAP prevention, we presented a cost-effectiveness analysis (CEA) of using HME to prevent VAP in mechanically ventilated patients in ICU (18-20).
2. Objectives
The aim of this study was to assess the incremental cost-effectiveness ratio associated with the use of HME filter to prevent VAP compared with HH presently adopted by ICU services within the Brazilian healthcare unified system.
3. Patients and Methods
This study consists of a CEA to compare the cost-effectiveness of HME and HH in preventing VAP (outcome) in mechanically ventilated adult patients admitted to an ICU. Because VAP is an acute condition, the simple decision tree model (one cycle per year) was used. The software TreeAge Pro 2011® was used to conduct CEA. The survey of costs and CEA were accomplished by the following recommendations described in methodological guidelines for studies on the economic assessment of health technologies (21) and in the studies of Nita et al. (22), Cartwright (23) and Hoch and Smith (24).
The ethics committee of hospital das clinicas da faculdade de medicina de Ribeirao Preto, University of Sao Paulo, Brazil (protocol 7076) approved this study.
3.1. Perspective of Analysis
The CEA was conducted from the perspective of a public university hospital that delivers highly complex and technological assistance to patients. The hospital is funded by the Brazilian unified healthcare system; the Sao Paulo State health secretariat also contributes to hospital funding.
3.2. Analyzed Time Frame
Because VAP is an acute episodic condition and since mechanically ventilated patient-hospital bed was the unit analyzed in this work, a period of one year was assessed. Costs recorded in 2011 were used as reference. Inflation rates and discounts were not used.
3.3. Target Population
The hospital complex where this study was undertaken delivers care to the population of four regional health departments (RHDs) in the State of Sao Paulo, Brazil. These RHDs include the cities of Ribeirao Preto, Franca, Araraquara and Barretos with 3350000 inhabitants. The hospital has 1100 beds as well as medium and high complexity outpatient clinics in all medical specialties. The present CEA compared the use of HME and HH in adults only (patients aged ≥ 18 years), so the target population was estimated at 2,847,500 inhabitants (85% of the total population). In 2011, 29438 patients (1.033% of the inhabitants) were admitted to the wards of the hospital complex; 910 of these patients (that is, 3.09% of patients admitted to the wards) were admitted to the ICU. Therefore, the target population was selected based on (i) the demand measured in 2011, (ii) a recently published clinical trial (12) and (iii) meta-analysis (13) comparing the effectiveness of HH and HME in preventing VAP.
3.4. Study Context
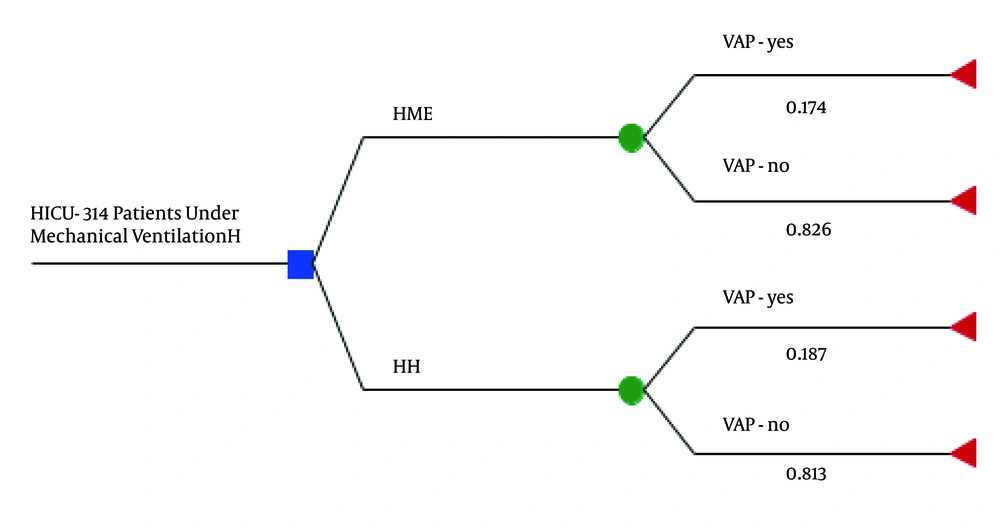
The CEA was initially conducted based on (i) a pre-post clinical trial reported by Auxiliadora-Martins et al. (12) and (ii) the results of a meta-analysis published by Niel-Weise et al. (13). The study by Auxiliadora-Martins et al. (12) involved 314 patients admitted to the ICU (nine beds) of the hospital complex studied herein; 146 and 168 patients used HME and HH, respectively. The study by Niel-Weise et al. (13) compared HME and HH for VAP incidence, which were 13.6% and 14.7%, respectively (effectiveness of 86.3% and 85.2%, respectively; P > 0.05).
The Incremental Cost-Effectiveness ratio (ICER) was calculated using the following (Equation 1):

Where C (HME) and C (HH) are the total cost related to the use of HME and HH, respectively and E (HME) and E (HH) are the effectiveness of HME and HH in preventing VAP, respectively.
3.5. Statistical Analysis
The data were presented using descriptive statistics. For the realization of cost-effectiveness analysis based techniques were used in appropriate guidelines for this type of study as described.
3.6. Basic Assumptions
3.6.1. Behavior of the Health Condition for Which the Target Technology Is Used
Acute event that culminates in admission to ICU regardless of underlying disease (the disease that motivated admission to the hospital complex) and which requires immediate MV due to decompensated comorbidities, accidents with subsequent trauma, intoxication, acute infectious diseases, post-surgical recovery and recovery from other procedures, among other reasons. ICUs in high-complexity hospitals have a 100% occupancy rate. Therefore, it is estimated that the number of mechanically ventilated ICU patients and the use of HME and HH technologies remain stable along the year (18, 19, 25, 26).
3.6.2. Restriction
The consensus among intensive care specialists and in literature papers is that HME is contraindicated in hemoptysis, thick airway secretion, carbon dioxide (CO2) retention and hypothermia, which correspond to 4% and 10% of patients admitted to general ICU (18).
3.6.3. Compliance
MV is a medical indication that offers vital support to patients who cannot decide on whether they wish to be placed under MV. Hence, compliance with MV and the technologies evaluated herein (HH and HME) is 100%.
3.6.4. Financial Funding
Financial funding is 100%.
3.6.5. Sensitivity Analysis
Univariate analysis was used; variations in the number of patients, MV (via HH or HME) duration and changes in VAP rates were considered.
3.6.6. Costs
The microfinancing method was used to survey the unit costs (patient-bed/day) ¥. Only direct payments were considered. Other costs involved in critical patient care, such as the time that nursing staff spends on HH handling and HME replacement, were not accounted. Reliable computation of these costs is difficult and the institution cost management center often calculates them via the absorption costing method. Approaching and considering costs from the bed-ventilator/day perspective would also be possible. However, the patient-bed/day approach reflects the reality more adequately, which is the reason why it was selected for this study. Table 1 lists all the elements considered in the composition of total cost.
| HH | HME-F | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Daily Cost /1st Year | Cost/1st Year | Daily cost /2nd Year | Cost /2nd Year | Daily Cost | Cost/1st Year | ||||
| Equipment | 1.56 | 569.40 | 1.56 | 569.40 | Filter | 12.00 | 12.00 | 4,380.00 | |
| Water 100 mL | 10.92 | 3,985.80 | 10.92 | 3,985.80 | Water and equipment | 0.00 | 0.00 | 0.00 | |
| Humidifierd | 7,500.00 | 20.50 | 7,500.00 | 0.00 | 0.00 | Humidifier | 0.00 | 0.00 | 0.00 |
| Sensor/ flowe | 142.00 X 4 | 1.55 | 565.75 | 1.55 | 565.75 | Sensor/flowf | 142.00 x 2 | 0.78 | 284.70 |
| Electric power/month | 16.42 | 0.55 | 201.48 | 0.552 | 201.48 | Electric power | 0.00 | 0.00 | 0.00 |
| Autoclave/month | 32.00 | 1.10 | 401.50 | 1.10 | 1.10 | Autoclave | 0.00 | 0.00 | 0.00 |
| Total | 36.18 | 13,205.70 | 15.68 | 5,723.20 | Total | 12.78 | 4,664.70 | ||
aSource: Institution cost management center.
bNote: In the case of HME-F, it is not necessary to differentiate the 1st and 2nd years because there are no purchase costs as compared with HH
cPatient-bed = bed-day maintenance costs associated with patient costs, including fixed and varied cost variables
dThe cost of the humidifier was accounted for in the first year (purchase) and was used for HH only. Cost in R$ based on 2011 dollar quotation (U$S 1.83).
eThe flow sensor has to be replaced every four months in the case of HH.
fThe flow sensor has to be replaced every six or more months in the case of HME-F. In the present analysis, replacement every six months was considered.
In the first year, costs related to the use of HH are clearly higher than the costs related to the use of HME, because it is necessary to purchase the humidifier. According to the institution price database for the year 2011, the humidifier costs R$ 7,500.00. The sensor, another component of the total cost, also translates into different costs between the two technologies, experimental studies (unpublished data) have shown that the sensor demands substitution every three months in the case of HH, whereas replacement must be performed every six months or even after longer periods for HME. The present CEA considered HME replacement every 24 hours; even though, studies have demonstrated that replacement every 48 hours is as safe as that of every 24 hours (19, 25). Besides, some reports indicated that replacement every seven days does not pose increased risks to patients (26, 27).
4. Results
The study by Auxiliadora-Martins et al. (12) was conducted in ICU of the hospital complex where the present investigation was performed; the study included patients under MV for over 48 hours, subdivided into HH group (168 patients, 53%) and the HME group (146 patients, 47%). Based on the aforementioned study, HH and HME patients presented similar clinical and sociodemographic characteristics; the compared variables did not differ significantly. At the end of study, the density of VAP incidence in the HH (29 VAP cases/168 patients) and HME (27 VAP cases/146 patients) groups were 18.7 and 17.4 cases/1000 MV days, respectively ([OR = 1.09; IC 95%: 0.61 - 1.94]; P > 0.05). MV lasted 11 and 12 days in the HH and HME groups, respectively (p > 0.05). Mortality in the HH and HME groups were 55.3% and 55.4%, respectively; the death risk calculated by APACHE score were 56% and 58%, respectively. The probability of contracting VAP was strongly associated with MV duration, even after the analysis was adjusted by the use of HME or HH. These data enabled construction of a simple decision tree model (Figure 1). The analysis considered a period of 12 months; MV duration of 11 and 12 days for patients in HH and HME groups, respectively and a daily cost of R$16.46 and R$13.42 for HH and HME, respectively. Table 2 Calculated Incremental Cost-Effectiveness Ratio (ICER). CER decreased more in favor of HME, as demonstrated in Figure 2.
Schematic Model (decision tree) for the Cost-effectiveness Analysis of the Use of HH and HME, Based on the Study by Auxiliadora-Martins et al. (12)
| Technology | Total Cost | Effectiveness, % | CER | dC | dE | ICER |
|---|---|---|---|---|---|---|
| HH | R$ 30,418.08 | 81.3 | R$ 37,414.62 | - | - | R$ 37,414.62 |
| HME | R$ 23,511.84 | 82.6 | R$ 28,464.70 | R$ 6,906.24 | 0.013 | R$ 531,249.24 |
Abbreviations: CER, cost-effectiveness ratio; dC, cost difference; dE, effectiveness difference.
aCost in R$ based on 2011 dollar quotation (U$S 1.83).
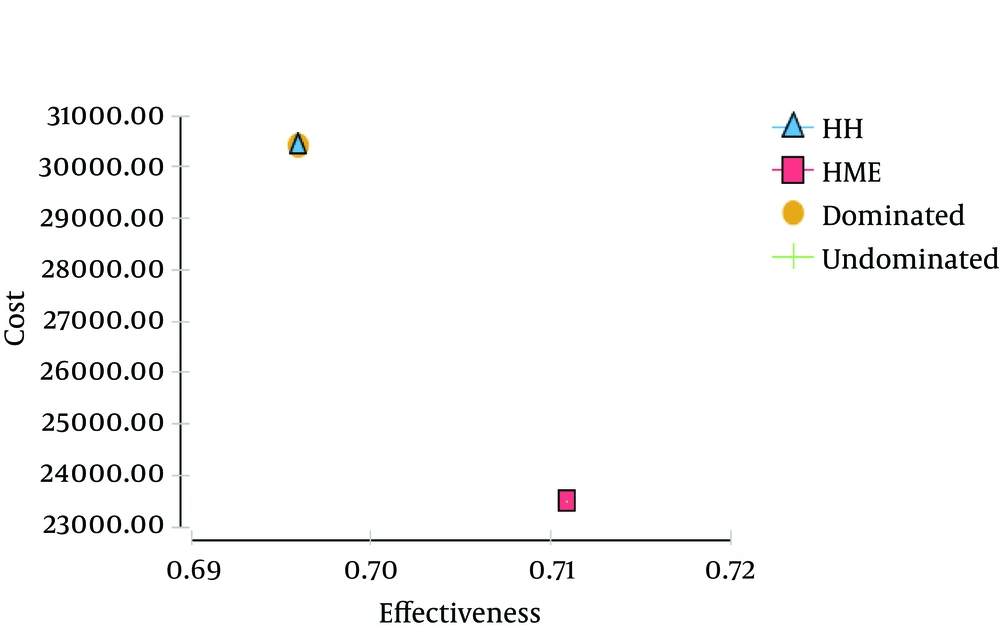
Again, HME was more attractive; costs ranged from R$ 21,000.00 to R$ 22,000.00 and effectiveness was close to 0.71, as compared with a cost of R$30,000.00 and effectiveness between 0.69 and 0.70 for HH. Based on previous assumptions, CER and ICER displayed in Table 3 were obtained.
| Technology | Total Cost | Effectiveness, % | CER | dC | dE | ICER |
|---|---|---|---|---|---|---|
| HH | R$ 30,418.08 | 81.3 | R$ 37,414.62 | - | - | R$ 37,414.62 |
| HME | R$ 21,552.52 | 82.6 | R$ 26,092.64 | -R$ 8,865.56 | 0.013 | R$ 681,966.16 |
Abbreviations: CER, cost-effectiveness ratio; dC, cost difference; dE, effectiveness difference
aCost in R$ based on 2011 dollar quotation (U$S 1.83).
4.1. Sensitivity Analysis
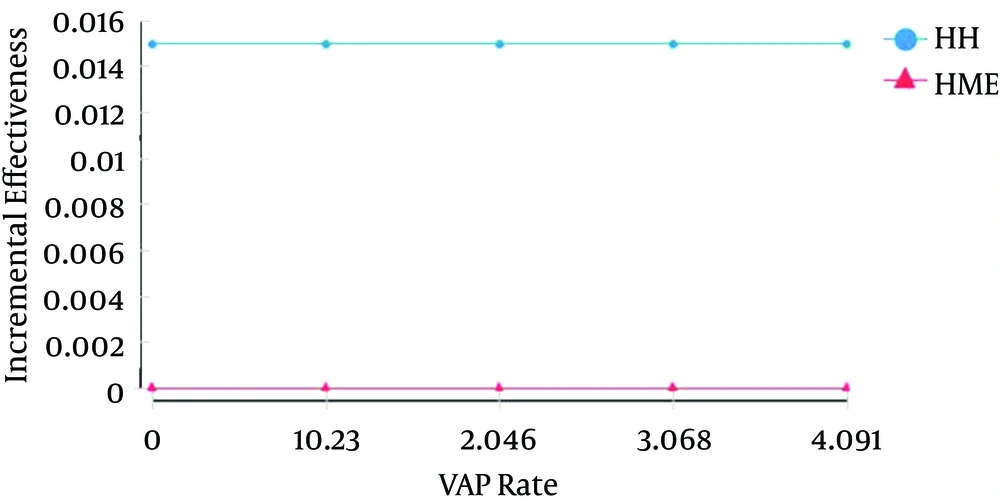
The four main parameters used during the univariate sensitivity analysis were as follows; The VAP (outcome) rate had minimum, reference and maximum values of 0, 0.174 and 4.09, respectively. The effectiveness of technologies in preventing VAP had minimum, reference and maximum values of 0.694, 0.711 and 0.712, respectively. As for the incremental cost, the minimum, reference and maximum values were R$ 0, R$ 6,906.24 and R$ 8,000.00, respectively. Concerning the mean cost-effectiveness, the minimum, reference and maximum values were R$ 32,000.00, R$ 33,000.00 and R$ 46,000.00, respectively. The unit and total costs were not included in the analysis, because they remained unaltered in real conditions. The first parameter to be tested was the VAP rate (Figure 3); it varied from 0 to 4.09/1000 MV days. Even after wide variation in the VAP rate, the costs remained constant and resembled the costs used in the CEA. The HME remained stable and lower than the HH costs.
The mean effectiveness and incremental effectiveness remained constant even though they depended on the VAP rate geometric progression. HME and HH differed significantly for the incremental effectiveness. Even after an effectiveness gain of 1.5% in favor of HH and despite the wide variation in the VAP rate, the HME effectiveness remained stable. The mean CER was also analyzed as a function of the geometric variation of the VAP rate. Upon large variations in this rate, the mean HME cost-effectiveness was lower than the mean HH cost-effectiveness, being the HME value close to R$ 44,000.00.
5. Discussion
In this study, we searched publications dealing with costs and economic assessment of the use of HME compared with conventional humidifiers (HH) in any context (pediatric or adult ICU, general anesthesia under MV). We used PubMed, Scopus/Embase, ScienceDirect, Cochrane, Scielo and Lilacs databases for this purpose. We found four studies (17, 26-28), in which all the analyses consisted of a survey of costs. These studies did not conduct cost-effectiveness, cost-benefit or cost-minimization analyses, among others and they did not follow updated methodological recommendations. All the retrieved publications dealt exclusively with clinical advantages and disadvantages of HME and HH and focused on well-defined clinical outcomes, especially VAP. Some studies made general reference to costs involved in the use of HME and HH. Results were always more favorable for HME, although no comparative methodology supported data. Therefore, the present study was probably the first to assess the cost-effectiveness of HH and HME.
HME was more cost-effective than HH in preventing VAP. VAP was the selected outcome, because it translates the large use of MV and is the most investigated outcome in clinical trials as well as systematic reviews with meta-analysis. The CEA conducted here was based on a recent clinical study (12) and on an even more recent meta-analysis (13), where the VAP rate was slightly lower (albeit without significance) in mechanically ventilated patients using HME compared with HH. Due to small difference between the effectiveness of HME and HH and lower costs of HME compared with HH, ICER was considerably favorable for HME (considering HME replacement every 24 hours). Nevertheless, ICER results for HME gave negative monetary amounts as a result of the small difference in the effectiveness of HH and HME for the investigated outcome (VAP prevention). In other words, the results predicted that HME offers a net monetary benefit and little or no net health benefit; i.e., HME undoubtedly has economic advantages. Because HH and HME have similar effectiveness, cost-minimization analysis (CMA) might be more suitable in this case; such analysis is based on Equation 2:

Where DCHH and DCHME are direct cost of using HH and HME, respectively, could provide data that are easier to interpret. In the first analyzed condition, CMA could be calculated as follows; CMA = R$ 30,418.08 - R$ 23,511.84 = R$ 6,906.24. Considering the reference study (12), the more recent meta-analysis (13) and comparison of HH and HME for the same outcome, with the same effectiveness pattern, this indicates that HH involves higher costs than HME. The CMA value would be even higher if HME was replaced every 48 hours, which would make this technology even more economically advantageous. In fact, in their eight-year monitoring of HME, Salemi et al. (27) noted that HME replacement every 48 hours or even weekly basis did not pose risks to mechanically ventilated patients, as long as recommended good ICU practices were followed. Indeed, this strategy represented considerable economy of financial resources in the context of a 16-bed ICU. Although HME do not prevent VAP (29, 30), in this study it demonstrated to be cost-effective.
5.1. Limitations of the Study
Although the present evaluation followed up-to-date recommendations for the analysis of cost impact, it presents limitations inherent to any study that applies modeling and simulations (scenarios). Some limitations are as follows:
The a priori variables used for cost analysis and indication of the target technology (HME-F) are well defined and informative (closed). These conditions make testing of the robustness of the model difficult, because this test would require simulation of a larger number of scenarios and broader sensitivity analysis. On the other hand, the use of MV is inherent to the ICU context and any technological assessment in terms of medications, procedures and equipment (as in the present study) is limited to well-defined variables with low variability, which leaves room for only a few possible scenarios applicable to real life.
It was not possible to observe common conditions expected for other technologies, such as variability in terms of compliance, since mechanically ventilated patients cannot choose not to comply with MV.
Another aspect to consider, albeit not a limitation, is 100% ICU occupancy rate in high-complexity and university hospitals. Most if not all ICU patients would be submitted to MV with HME-F or HH. In this case, costs may have a different impact compared with smaller hospitals.
The perspective of the present analysis was a public hospital funded as follows; the Brazilian unified health system provides 25 to 28% of the financial resources, whereas the Sao Paulo State health secretariat guarantees most of the funding. Therefore, the real impact on the budget is lower than the impact presented here.
5.2. Conclusion
Although ICER favored the use of HME, the values were negative. These results revealed that HH and HME differ very little in terms of effectiveness, which makes interpretation of the results in the context of clinical practice difficult. Nonetheless, there is no doubt that HME is advantageous. This technology incurs lower direct cost. Another aspect is the indication to substitute HME every 48 hours, which reduces costs and impacts of ICER even further.