1. Background
These days, the trend of pharmacotherapy is toward controlling diet instead of drug administration. Amino acids have been shown to have numerous different effects and thus can be used in various medical situations such as inflammation (1-3), tumor therapy (4), trauma (5, 6), etc. The antinociceptive effect of different peptides and amino acids was indicated recently (7, 8); yet, there is a lack of detailed information on their mechanism.
Creatine (Cr) and Whey (Wh) are amongst the most favorable supplements on the market, which are rich sources of amino acids (9, 10). However, it should be considered that amino acids are rarely used solely and probable interactions between them may result in different effects. Two probable mechanisms of analgesic effect of peptides are inhibition of prostaglandin synthesis and their effect on analgesic opioid receptors (11). Also, one of the important effects of these products is augmentation in nitric oxide (NO).
The analgesic characteristics of ketamine have been determined, yet the exact mechanisms are unknown. Morphine can be inhibited by NO. N-Methyl-D-aspartate (NMDA) antagonist can inhibit this effect of NO. Therefore, there may exist a relationship between NO and NMDA receptors. Both whey and creatine can augment the plasma level of NO (12, 13), yet the effect of NO on NMDA antagonist analgesia is still ambiguous.
2. Objectives
The main question of this study was whether this augmentation in NO level is effective on analgesic and anesthetic effect of ketamine (NMDA antagonist), and whether this is related to the pure increase of NO (creatine) or the peptides and amino acids (which exist in whey). We investigated the probable role of Cr and Wh by using ketamine anesthesia, which is a non-competitive antagonist of NMDA (14). Also we examined the probable changes of Cr and Wh supplement consumer which could be present in their anesthesia.
3. Materials and Methods
Thirty male Wistar rats that weighted 250 ± 30 grams were obtained from the laboratory animal center of Tehran University of Medical Sciences (TUMS). The rats were let to become accustomed to the experimental environment within a week. Prior to the gavage period, all the rats became familiar with human touch for 30 minutes for five days. In our experiment, male Wistar rats were chosen in order to minimize hormone interaction with the final results. The rats were divided to three main groups (n = 10): whey group, creatine group and sham group (gavage with water only). The dosage and protocols used for gavage are described later. Suitable protocols were chosen according to guide for the care and use of lab. Animals (15) and project ethics were accepted by the ethics committee of the research center of TUMS.
3.1. Nutrition and Rat Gavage
Standard rat food (Table 1) was utilized to feed the rats. To reach maximum stomach distention before gavage, the rats were not fed three hours prior to the start of the gavage process. The maximum liquid used for every individual was 1 mL/100 gr of body weight. Whey protein of Karen nutrition co. (nutrition value mentioned in Table 2) was used at 1.9 g/day for a rat that weighed about 250 g; this dosage is equal to an ordinary intermediate human dosage of 30 g/day (16). To adopt the present dose with the rat’s weight, we used the Reagan Shaw method.
| Ingredients | Valuesa |
|---|---|
| Pure protein | 13 |
| Min. ash | 10 |
| Salt | 0.5 - 0.55 |
| Methionine | 0.33 |
| Tryptophan | 0.25 |
| Pure fat | 3.5 - 4.5 |
| Calcium | 0.95 - 1 |
| Pure fiber | 4 - 4.5 |
| Phosphor | 0.65 - 0.7 |
aData are presented as percentage.
| Ingredients | Values |
|---|---|
| Protein, g | 25 |
| Total fat, g | 0.4 |
| Saturated fat and cholesterol | 0 |
| Carbohydrate, g | 0.3 |
| Sugar, g | 0.3 |
| Sodium, mg | 30 |
The creatine used in this research was creatine monohydrate of Karen nutrition co. that was utilized at two different dosages of loading and maintenance. The loading dose was 0.430 g/kg during the first five days; the process was continued for four more weeks, five days a week with a maintenance dose of 0.143 g/kg (17). This biphasic dosage is the standard safe approved dosage for this supplement. Because solubility of the powder form of creatine is dependent on water temperature, we needed to use an optimized temperature for water to dissolve the powder. After termination of gavage, the rats were placed in their cage and standard food was provided. The process of gavage was done for 25 days.
3.2. Blinding Process
Before preparing the rats for anesthetization, we had to design a protocol to provide a triple blind project. All the cages were put in a room and one of the colleagues entered the room, displaced the cages and changed their label to alphabetic characters. Then, the first colleague exited and the next one performed the process again with numbers. The data that was sent for analysis, was sent with this form.
3.3. Anesthesia and Analgesia
All the rats were gavaged two days before anesthesia. Every day, six rats underwent anesthesia. For anesthetization, an insulin needle was used to inject rats, intraperitoneally, with 70 mg/kg of ketamine and 7 mg/kg of xylazine in the right lower quarter of their body. These dosages are known as intermediate doses for anesthetizing rats.
One hour before anesthesia, the rats were not fed to standardize their glycogen reserve. Their whole fast time with time of anesthesia did not exceed five hours; however, to provide an adequate body perfusion and cerebral protection, moderate hypothermia (32-34°C) was established throughout the anesthesia process. Just after injection of ketamine/xylazine combination (KX), we started a chronometer until the rat was anesthetized; this time was documented as the delay time. One minute after injection, the rats were rolled to their sides every minute until loss of righting reflex; this was assumed as commencement of anesthesia. One minute after that, a forelimb pinch was adjusted, using a ¾ inch binder clip. If withdraw reflex was present, the next pinch was established in the hind limb, one minute after the last one. This was done alternately between the forelimb and hind limb until loss of pain reflex. Although the clips were safe enough, these pinches were established on the metacarpal and metatarsal, in order to provide maximum protection. Of note, at the same time, the clip pressure was enough to make a withdraw reflex. After recording the loss of pain reflex time, we waited until the rats achieved the sternal position and after three consecutive resistances to rolling, anesthesia termination time was recorded. The “total sleep time” was the time between loss of righting reflex and anesthesia termination.
4. Results
Data were analyzed using the SPSS 18 software. With respect to the blind protocol, we performed our test twice. First time the rats that hadn’t become anesthetized were excluded; there was only one rat in our sham group with this situation. Also, two rats from the same group didn’t become painless although one of them had become anesthetized.
In this case, there was no data with a significant P-value. The second time, we performed our test among all the rats and none of them was expunged. This time only the “total anesthesia time” was significant (P < 0.05) using Analysis of Variance (ANOVA) Post hoc test. Descriptive details of the analysis are presented in Table 3.
| Groups | Valuesa | P Value |
|---|---|---|
| Delay time | .313 | |
| Creatine | 14.5020 ± 15.07169 | |
| Whey | 5.3630 ± 4.83643 | |
| Sham | 8.9229 ± 16.56205 | |
| Total | 9.5957 ± 13.32362 | |
| Time to forelimb analgesia | .568 | |
| Creatine | 26.2380 ± 28.71558 | |
| Whey | 17.4310 ± 17.83910 | |
| Sham | 14.9780 ± 25.98641 | |
| Total | 19.5490 ± 24.25741 | |
| Time to hind limb analgesia | .553 | |
| Creatine | 27.5650 ± 28.72908 | |
| Whey | 18.0840 ± 17.67082 | |
| Sham | 16.3080 ± 26.04358 | |
| Total | 20.6523 ± 24.26543 | |
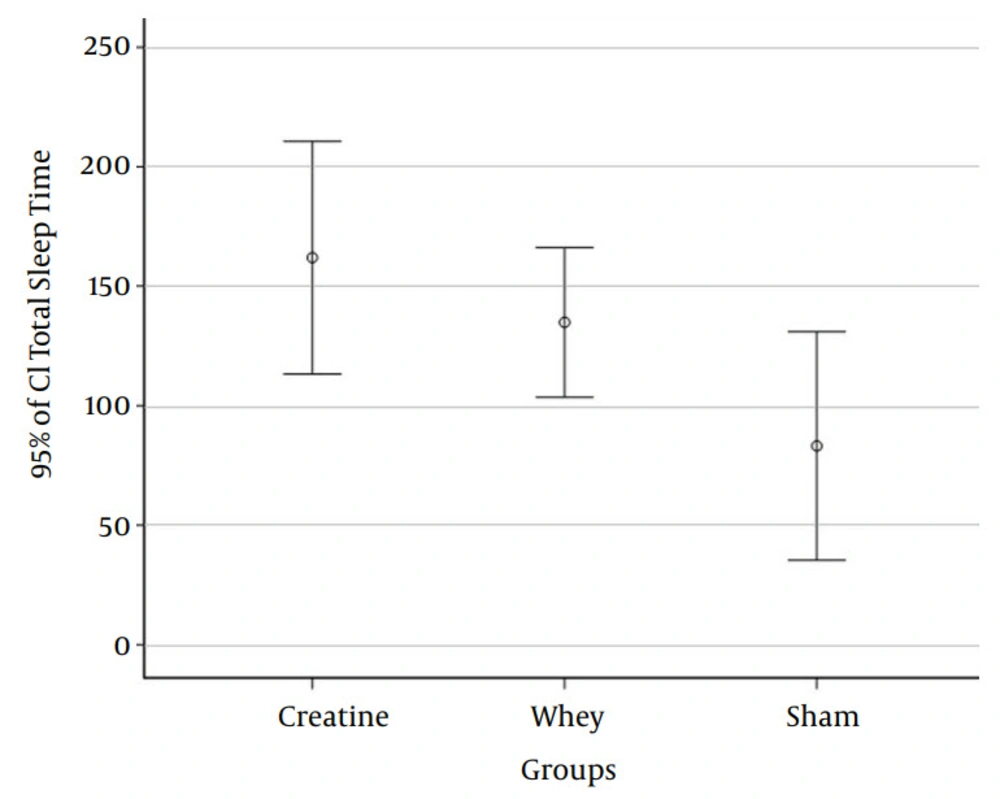
| Total sleep time | .23 | |
| Creatine | 162.00 ± 68.007 | |
| Whey | 134.90 ± 43.712 | |
| Sham | 83.20 ± 66.826 | |
| Total | 126.70 ± 67.228 |
aData are presented as mean ± SD.
To ensure that the group difference was causing such significance, we performed a Bonferroni multiple comparison test. The mean difference between the creatine and sham group (P < 0.021) was 78.80 minutes. Figure 1 shows the difference among the groups.
5. Discussion
Whey is a protein extracted from milk. Milk consists of two main proteins: whey (20%) and casein (80%). Whey is a water-soluble protein that can be digested rapidly in the body. Different researches have shown that it can make a positive nitrogen balance; that can cause muscle hypertrophy, increasing recovery speed after exercise and also enhancing the function of the immune system (18-20). Different types of whey are present on the market, which are classified due to their hydrolysis level. Usually, 90% of isolated whey protein volume consists of proteins.
In fact, whey protein is a combination of different amino acids (essential, non-essential and Branched Chain Amino Acids (BCAA)). Also, in some trade types, different vitamins and minerals (such as vitamin c, niacin, E, B6, B1, B2, Biotin, Folic acid, etc.) are being utilized. In our research, to minimize different component disturbances, we utilized products that did not have significant vitamins and minerals. The protein we used was Isowhey powder that was produced under microfiltration cross flow at low temperatures. It consisted of peptides, tripeptides, oligopeptides and combination of enzymes (protease 1 and 2, lactase and amylase), natural and artificial flavors and sucrose. The enzymes that are used in this whey were utilized to provide a more rapid and better digestion and according to previous researches do not make any interference with the research results. Micro fractions of the most important ingredients of whey protein are presented in Table 4.
| Wh Micro Fraction | Values |
|---|---|
| Beta-lactoglobulin (β-lg) | 45 - 50 |
| Alpha-lactalbumin (α-lac) | 15 - 20 |
| Bovine Serum Albumin (BSA) | 5 - 6 |
| Immunoglobulins | 10 |
| Lactoferrin | 1 |
| Lactoperoxidase | 1 |
| Glycomacropeptide | 15 |
As mentioned previously, whey protein consists of different amino acids. Essential amino acids are valine, tryptophan, histidine, methionine, phenylalanine, Threonine, isoleucine, leucine and lysine. Non-essential amino acids are alanine, glutamine, asparagine, cysteine, proline, glycine, tyrosine, serine, arginine, aspartic acid and glutamic acid (20). However, from this list, some of them are known as BCAA (leucine, isoleucine, valine) (21), which are important in muscular mechanisms and have many anabolic effects. They are absorbed very quickly and that is a result of metabolization in muscles instead of liver. It was proved that in addition to their NO retention ability, some of the components listed above (especially arginine (22)) are important precursors of NO (23). It is believed that NO has some inflammatory, neurotransmitery and immune system regulatory effects (22) and it is also involved in Alzheimer’s, heart disease and some other disorders. The inhibitory effects of NO in morphine analgesia are believed to be ceased by a NMDA antagonist (12).
Ketamine is a noncompetitive NMDA antagonist, which is one of the most usual anesthetics for use in animals. This drug is mostly used, along with xylazine, which is an alpha-adrenergic drug with analgesic, muscle relaxant and sedating supplementary effects (14). Our dosage of 70 mg/kg of ketamine plus 7 mg/kg xylazine is known as an intermediate dose for suitable anesthesia and analgesia; however, there is always a possibility of individual differences between the rats. In addition, it should be declared that because the utilization of xylazine was at the same dosage among the rats, it can’t be considered as interference for our study. Despite other analgesics, ketamine increases cerebral blood flow and cerebral metabolic rate of O2 with dilation of brain venous flow (24). Ketamine is metabolized in the liver and is among scarce anesthetics that have a really low albumin band (25). A comparison between isoflurane, KX and combination of medetomidine, midazolam and fentanyl, indicated that KX was the safest drug regarding hemodynamic changes and body temperature, though there was a prolonged wake-up and recovery period (26). To find a probable relationship between anesthesia and these supplements, we should also consider the important effects of these amino acids, including growth hormone release, depression control, involvement in the immune system and muscle growth, energy production, augmentation of blood sugar, inhibition of muscle atrophy, anti-oxidant properties in tissues, diminution of inflammation and anti-aging properties (20, 27). Some other clinical usages of our supplements (which are sources of amino acids) include involvement in skeletal metabolism (28, 29), tumor control, immunity system amelioration (18, 19), trauma and critical situation (5, 6, 30, 31), sepsis (32), anti-inflammation (1, 2) etc.
Whey protein with creatine is among the most popular supplements that athletes consume (10, 33). Reasons behind the use of these supplements are power maintenance, doctor’s recommendations and increase of body resistance during exercise. Researches have indicated a common reason for the utilization of whey and creatine, which is the augmentation of power. To discern the reality of this claim we should become familiar with creatine.
Creatine monohydrate, used in the present study, is a natural material, which changes to creatine phosphate in the body. Creatine phosphate, itself, can lead to form Adenosine Triphosphate (ATP), which provides enough energy for muscular activity (34). Naturally, the body can produce creatine and can also obtain it from nutrition (like red meat and fish) (20). In the liver and kidneys, creatine can be produced from arginine, glycine and methionine. About 95% of produced creatine, enters the blood circulation and can be stored in heart cells and body cells.
Because of creatine popularity, there were vast investigations about creatine effect on health status. Briefly, these researches have shown that taking creatine can result in diminution of homocysteine level in the body (23). Increased level of homocysteine can provide many psychological and neuronal disorders, which can lead to depression, dementia and Alzheimer. In fact, studies have shown that augmentation in homocysteine level may multiply the chance of Alzheimer disease by two. Also, it has been claimed that creatine has significant antioxidant effects. Furthermore, creatine can decrease the incidence of coronary heart disease (CHD) with diminution in homocysteine level. To the best of our knowledge, this was the first study, which investigated the relationship between whey, creatine and ketamine anesthetic and analgesic status.
Gavage of this volume of supplement needed to be done cautiously and precisely, especially for creatine, which is less soluble in water. The process should be performed gently by experienced researchers to avoid high rate of mortality and morbidity. Accordingly, there was no mortality in the current study. Our study didn’t show any significant difference in analgesia status between the different groups yet yet data shown that it may exist a difference between Cr consumers’ anesthesia and other groups; indeed,their sleep time was longer. However, further studies with different genders and dosages are required to decrypt the effect of these supplements on anesthesia and analgesia.