1. Background
Acetaminophen is one of the most commonly used drugs worldwide for treatment of fever and pain (1, 2). It was first synthesized in 1878 by Morse, and was introduced for medical usage in 1887 by von Mering (1, 2). However, despite being in use for more than a century, the mechanism underlying its therapeutic effects remains unclear. At least one study has suggested the possibility that the site of action of acetaminophen’s analgesic and antipyretic effects can be manipulated in the central nervous system (3). It has recently been demonstrated that, following deacetylation to p-aminophenol in the liver, acetaminophen is metabolized by fatty acid amide hydrolase (FAAH) to form N-(4-hydroxyphenyl) arachidonamide (AM404) in rat and mouse brains (1). AM404 acts as a potent activator of transient receptor potential cation channel subfamily V member 1 (TRPV1), as a ligand at cannabinoid CB1 receptors (4), or as an inhibitor of anandamide uptake, which is an endogenous agonist for both TRPV1 and CB1 receptors, subsequently increasing the concentrations of endogenous agonists (5-8). A number of studies have shown that acetaminophen is metabolized to AM404 in both rat and mouse brains by FAAH; however, these results were based on the use of acetaminophen concentrations higher than those used clinically in humans (15 - 20 mg/kg for adults) (5, 9). In addition, the conversion rate of acetaminophen to AM404 at these clinical doses has not yet been determined. Assuming that AM404 is the active substance of acetaminophen, estimation of AM404 concentrations in rat plasma is important.
2. Objectives
The aim of this study was to examine the metabolism of AM404 from acetaminophen in the rat brain at a concentration of 20 mg/kg, which is used in therapeutic practice in humans, and to compare the pharmacokinetic parameters of acetaminophen and its metabolites, including AM404.
3. Materials and Methods
3.1. Animals
The experiments were conducted in accordance with the appropriate bylaws (IACUC No. 10050 and 10052) of the Institutional Animal Care and Use Committee (IACUC) of Shin Nippon Biomedical Laboratories, Ltd. Six-week-old male Crl:CD(SD) rats (Charles River Laboratories Japan, Yokohama, Japan) were acclimated in rooms with controlled temperature (22 ± 3°C) and humidity (50% ± 20%) with a 12 hour light/dark cycle, for one week before use. Rats were allowed ad libitum access to tap water and rodent diet (CE-2, CLEA Japan, Inc.).
3.2. Chemicals
Acetaminophen and AM404 were obtained from Tyco Health Care Japan (Tokyo, Japan) and Enzo Life Science, Inc. (New York, USA), respectively. Acetaminophen-d4, AM404-d4, 4-acetamidophenyl β-D-glucuronide sodium salt, 4-acetaminophen sulfate potassium salt, 3-cysteinylacetaminophen trifluoroacetic acid salt, 3-(N-acetyl-L-cystein-S-yl) acetaminophen sodium salt, 4-acetamidophenyl β-D-glucuronide-d3 sodium salt, 4-acetaminophen-d3 sulfate, 3-cysteinylacetaminophen-d5 (major) trifluoroacetic acid, and 3-(N-acetyl-L-cystein-S-yl) acetaminophen sodium salt-d5 (major) were obtained from Toronto Research Chemicals, Inc. (Ontario, Canada).
3.3. Treatment
Acetaminophen was dissolved in H2O (10 mg/mL) and orally administered to rats at a dosage of 20 mg/kg with a disposable syringe. Following the administration of acetaminophen, the rats were exsanguinated from the abdominal aorta under isoflurane anesthesia at 5, 15, 30, 60, and 120 minutes. Blood samples were collected at these time-points and centrifuged at 1800 g for 15 minutes at 4°C to prepare plasma. The brains were immediately removed and weighed after blood sampling. The whole brain was homogenized (POLYTRON® PT3100, KINEMATICA, Littau, Switzerland) with an equivalent weight of water in an ice bath, and stored at -70°C until analysis.
3.4. Determination of AM404 Concentration in Brain Homogenate Samples
Ten microliters of methanol and 1 mL of internal standard solution containing 50 pg/mL AM404-d4 were added to 300 µL of brain homogenate samples. The mixtures were centrifuged at 2000 g for 5 minutes at 4°C, and the supernatant was transferred to another tube and mixed with 1 mL of H2O. The supernatant was then loaded onto an Oasis MAX (60 mg, 3 cc) extraction cartridge (Waters, MA, USA), which was pre-activated with 3 mL of methanol, acetonitrile, and H2O. The cartridges were subsequently washed with 3 mL of 0.2% (v/v) acetic acid, 70% (v/v) methanol, and 50% (v/v) acetonitrile. After the compound was eluted with methanol, the eluent was evaporated under a stream of nitrogen. The residue was dissolved in 100 µL of 75% (v/v) methanol, then centrifuged at 20000 g for 10 minutes at 4°C. Next, 20 µL of the supernatant was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chromatography was performed using a Luna 3 µm PFP(2) 100 Å (100 mm, 2 mm, 3 µm; Phenomenex, CA, USA) and an HPLC system consisting of a Shimadzu 20A series instrument (Shimadzu, Kyoto, Japan). All runs were performed with the column thermostat set to 40°C at an elution flow rate of 0.3 mL/minutes, using 0.1% acetic acid (solvent A) and 0.1% acetic acid methanol (solvent B) as mobile phases. The analytes were separated using the following gradients: 0.0 – 5.0 minutes, 80% to 100% solvent B; 5.0 – 7.5 minutes, 100% solvent B; 7.5 – 7.6 minutes, 100% to 80% solvent B; and 7.6 – 10.0 minutes, 80% solvent B. The analyte and internal standard were detected with a Sciex API 5000 mass spectrometer (Applied Biosystems, Foster City, CA, USA) in negative ion mode, using a TurboIonSpray source at 450°C. The collision gas was set at level 4. Mass-to-charge ratios (m/z) were monitored from 394.4 to 134.0 for AM404 and from 398.4 to 138.0 for the internal standard, at a collision energy of −45 eV. The lower limit of quantification was 2 pg/mL for AM404. The standard curve was linear from 2 to 1000 pg/mL for AM404 (data not shown).
3.5. Determination of Concentrations of Acetaminophen and Its Metabolites in Rat Pplasma Samples
Twenty microliters of methanol and 100 µL of the internal standard solution, containing 200 ng/mL acetaminophen-d4, acetaminophen glucuronide-d3, acetaminophen sulfate-d3, cysteinylacetaminophen-d5, and acetaminophen mercapturate-d5, were added to 20 μL aliquots of the plasma samples, then mixed. After centrifugation at 15000 g for 5 minutes at 4°C, 10 µL of the supernatant was added to 200 μL of H2O, and the aliquot of the mixture was analyzed with LC-MS/MS. Chromatography was performed using a Capcell Pak MGII column (particle size, 5 μm; 2.0 mm I.D. × 100 mm; Shiseido, Tokyo, Japan) and a Shimadzu 20A HPLC system (Shimadzu, Kyoto, Japan). All runs were performed with the column thermostat set to 40°C and an elution flow rate of 0.3 mL/min, using 0.1% acetic acid and methanol as mobile phases. The initial mobile phase was 5% methanol, which was increased by linear gradient to 20% starting at 3 minutes. Detection of the analyte and internal standard was performed using a Sciex API 4000 mass spectrometer (Applied Biosystems, Foster City, CA, USA) in positive ion mode with a TurboIonSpray source at 400°C. The collision gas was set at level 4. Mass-to-charge (m/z) values were monitored from 152.0 to 110.1 for acetaminophen, from 328.1 to 152.1 for acetaminophen glucuronide, from 232.2 to 152.2 for acetaminophen sulfate, from 271.1 to 140.1 for cysteinylacetaminophen, from 313.1 to 208.2 for acetaminophen mercapturate, from 331.2 to 155.1 for acetaminophen glucuronide-d3, from 235.2 to 155.2 for acetaminophen sulfate-d3, from 152.0 to 110.1 for acetaminophen, from 276.1 to 143.1 for cysteinylacetaminophen-d5, and from 318.1 to 212.2 for acetaminophen mercapturate-d5, at collision energies of 22, 16, 22, 32, 25, 22, 16, 22, 32, and 25 eV, respectively. The lower limit of quantification was 0.05 µg/mL for all analytes. The standard curves were linear from 0.05 to 50 µg/mL for all analytes (data not shown).
4. Results
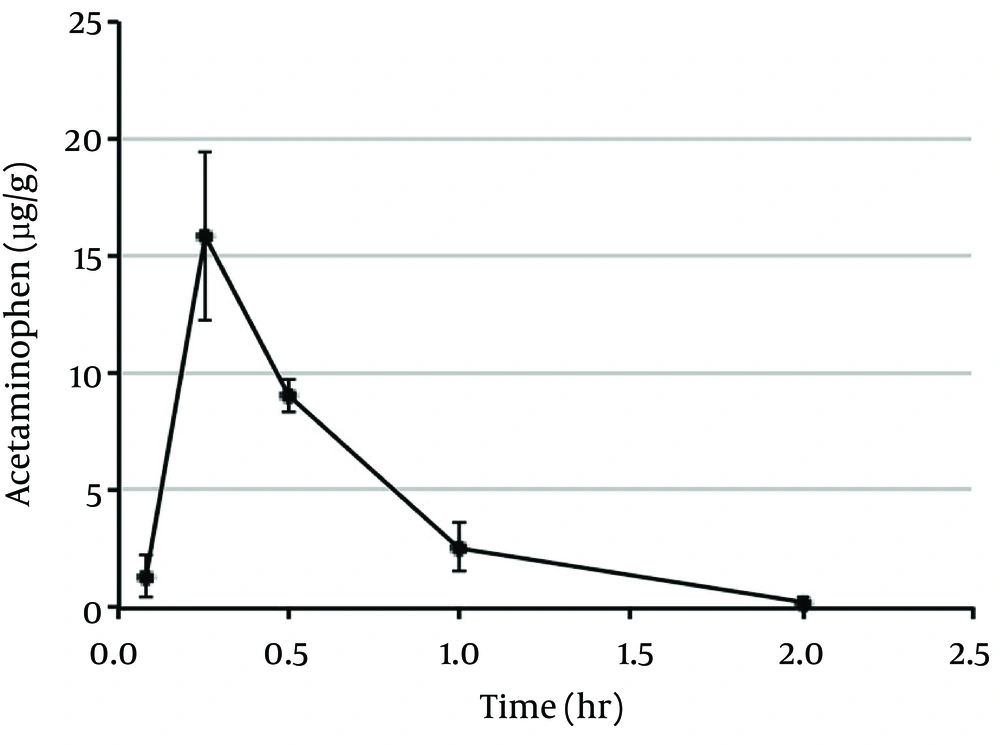
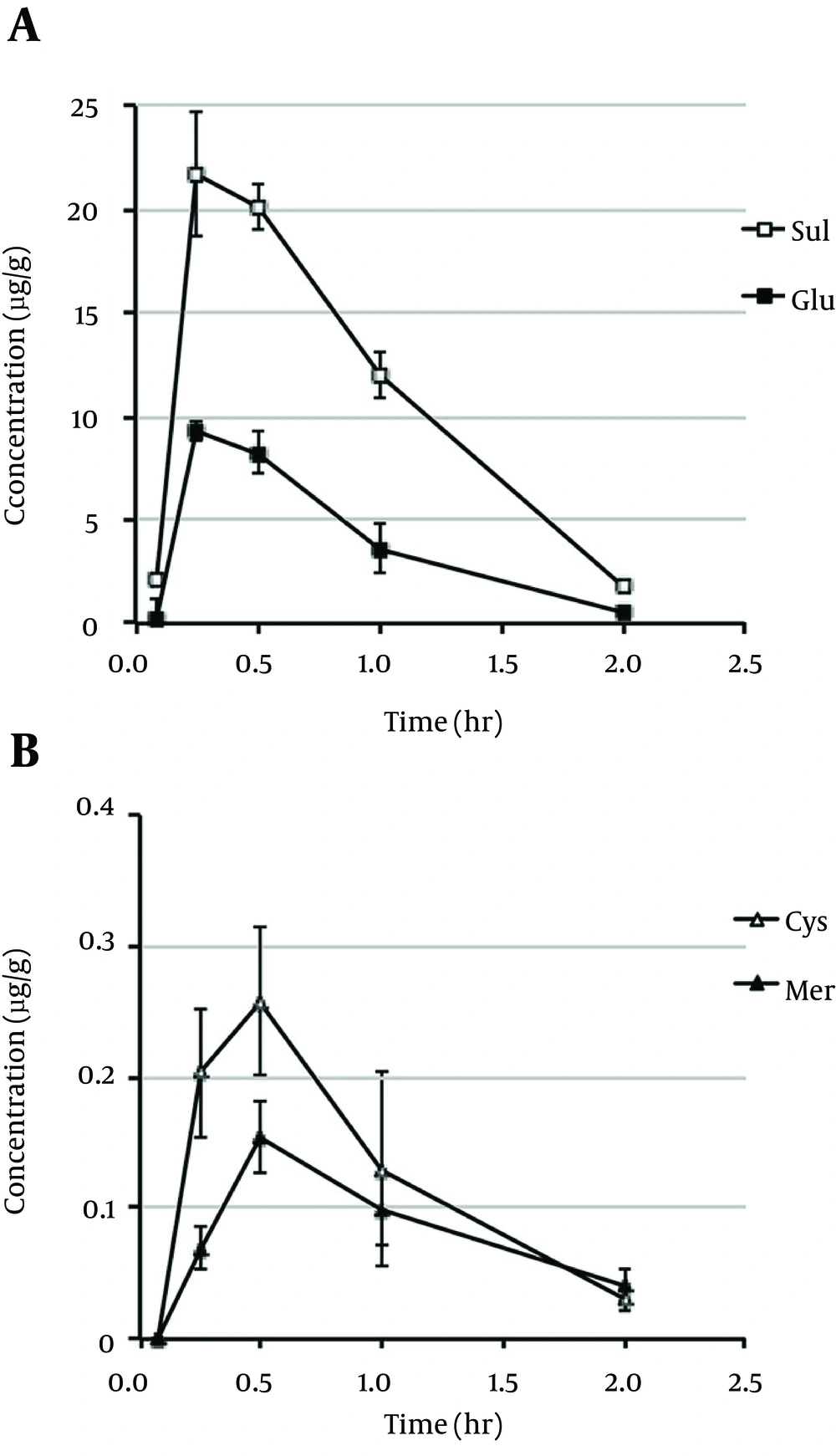
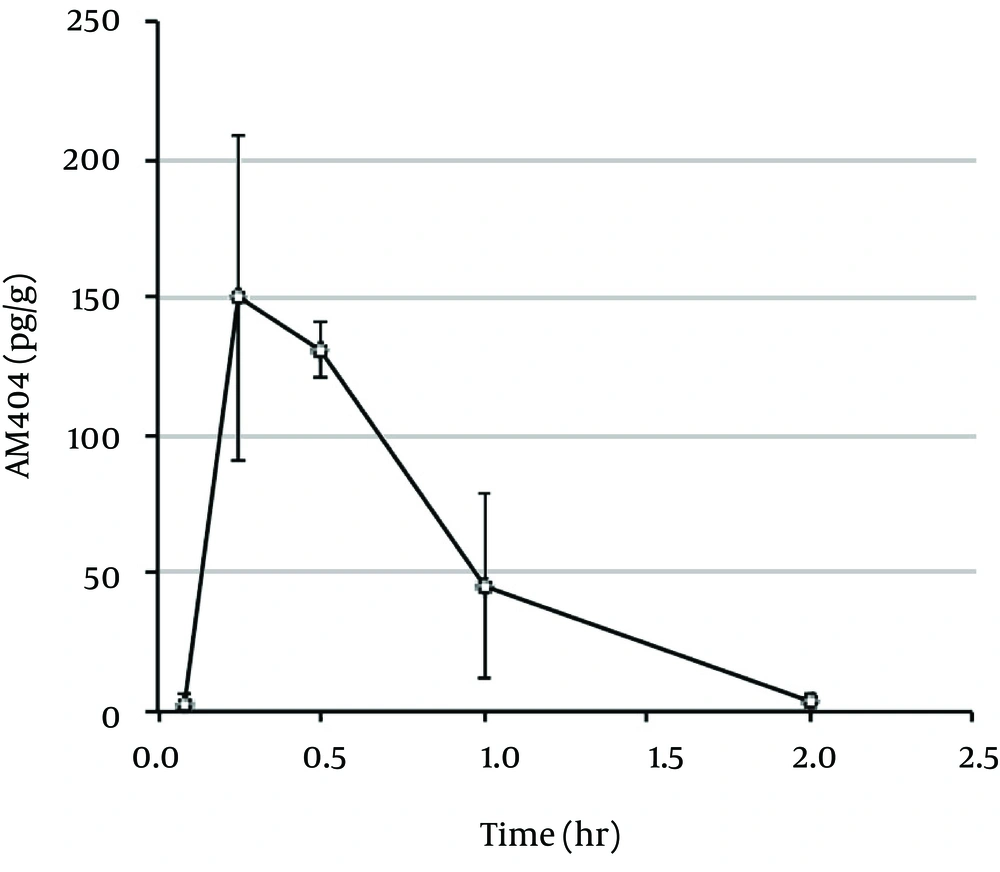
In the present study, the mean plasma concentration-time profiles and pharmacokinetic parameters of acetaminophen in rats were calculated following oral administration of 20 mg/kg doses (Figure 1 and Table 1). The mean plasma concentration-time profiles and pharmacokinetic parameters for the two major metabolites of acetaminophen-acetaminophen sulfate (Sul) and acetaminophen glucuronide (Glu) are shown in Figure 2A and Table 1. Acetaminophen cysteine (Sys) and acetaminophen mercapturate acid (Mer), which were metabolized from N-acetyl-p-benzoquinone-imine (NAPQI) after conjugation with glutathione, are also shown in Figure 2B and Table 1. The plasma concentration curves of acetaminophen, Sul, Glu, Sys, and Mer indicated rapid, monophasic decline, with t1/2 values of 0.298, 0.361, 0.416, 0.483, and 0.779, respectively. The maximum plasma concentration (Cmax) and the peak time to reach Cmax (Tmax) of acetaminophen were 15.8 µg/g and 0.25 hour, respectively (Table 1). The Tmax value was the same for Sul and Glu (0.25 hour) and for Sys and Mer (0.5 hour). The value of the area under the plasma concentration-time curve (AUC0-2h) of acetaminophen was 8.96 μg hour/g. Figure 3 shows the mean brain concentration-time profiles and pharmacokinetic parameters of AM404 following oral administration of acetaminophen to rats at 20 mg/kg. The curve representing the brain concentrations of AM404 declined rapidly in a monophasic manner, with t1/2 of 0.305 hour (Table 2). The Cmax, Tmax, and AUC0-2h values of AM404 were 150 pg/g, 0.25 hour, and 117 pg hour/g, respectively (Table 2). Although efforts were made to measure the concentration of AM404 in plasma, these levels were undetectable under the experimental conditions applied (data not shown).
| Cmax (µg/g) | tmax (hr) | t1/2 (hr) | AUC0-2h (µg hr/g) | Kel (1/hr) | |
|---|---|---|---|---|---|
| APAP | 15.8 | 0.250 | 0.298 | 8.96 | 2.32 |
| Glu | 9.30 | 0.250 | 0.361 | 7.99 | 1.92 |
| Sul | 21.7 | 0.250 | 0.416 | 22.2 | 1.67 |
| Sys | 0.258 | 0.500 | 0.483 | 0.251 | 1.44 |
| Mer | 0.154 | 0.500 | 0.779 | 0.167 | 0.890 |
aAbbreviations: APAP, plasma pharmacokinetic parameters of acetaminophen; Glu, acetaminophen glucuronide; Mer acetaminophen mercapturate acid; Sul, acetaminophen sulfate; Sys, acetaminophen cysteine.
bNote: Each value represents the mean concentration in rat plasma.
Plasma concentration-time profiles of acetaminophen sulfate (sul) (open square) (A), acetaminophen glucuronide (glu) (closed square) (A), acetaminophen cysteine (sys) (open triangle) (B), and acetaminophen mercapturate acid (mer) (closed triangle) (B) in rats following oral administration of 20 mg/kg acetaminophen. Each Point Represents the Mean ± SD of data obtained from three rats.
| Cmax (pg/g) | tmax (hr) | t1/2 (hr) | AUC0-2h (pg hr/g) | Kel (1/hr) | |
|---|---|---|---|---|---|
| AM404 | 150 | 0.250 | 0.305 | 117 | 2.27 |
5. Discussion
In the present study, we report the pharmacokinetics of AM404 in the rat brain following oral administration of acetaminophen at 20 mg/kg, which corresponds to the clinical doses used in humans. Hogestatt et al. showed that acetaminophen is metabolized into p-aminophenol, which can be further transformed into AM404 in the brain following FAAH-dependent conjugation with arachidonic acid at dosages ranging from 30 to 300 mg/kg (5). Acetaminophen dosages greater than those used clinically in humans (15 - 20 mg/kg for adults) may cause severe liver damage (10); therefore, accurate determination of the AM404 concentration-time profile at a clinical dosage is important for clinical use. To this end, we investigated the pharmacokinetics of AM404 at an acetaminophen concentration of 20 mg/kg, which is equivalent to the clinical dose used in humans (1). The mean peak brain concentration of AM404 (Cmax, 150 pg/g) was lower than the previously reported concentration of N-arachidonoylethanolamine (anandamide) in the rat brain (1494.4 pg/g) at physiological levels (11). Since acetaminophen does not exhibit strong analgesic effects and is effective only at high concentrations, it seems reasonable to conclude that this low concentration of AM404 (150 pg/g) plays an important role in analgesic signaling in the brain. The conversion rate of acetaminophen to AM404 was 0.0013%, based on the calculations performed using AUC0-2h. Even with this small concentration, analgesic effects may be observed with AM404 metabolized from acetaminophen at clinical dosages. The elimination half-life (t1/2) value of AM404 is similar to those of acetaminophen and its metabolites. These results support the idea that AM404 maintains a significant concentration in the brain while acetaminophen continues to exhibit effective plasma levels. The similarity in pharmacokinetics between AM404 and acetaminophen suggests that AM404 may be one of the important metabolites involved in the analgesic mechanism in the brain. However, we were unable to detect AM404 in the rat plasma (data not shown), as was reported by Hogestatt et al. (5). These results also suggest that the site of action of acetaminophen could be in the central nervous system. This is further supported by previous reports that suggest that TRPV1 in the brain, co-expressed with FAAH, is involved in the antinociceptive action of acetaminophen (9). The Cmax value of acetaminophen (15.8 μg/g) in the rat plasma was almost equal to that reported by Shinoda et al. after oral administration of a 1 g dose to healthy adult volunteers (17.7 μg/mL) (12). Therefore, the administration of acetaminophen at a dose of 1 g for an adult human, which is within the range of clinical concentrations used, is expected to produce AM404 concentrations in the human brain similar to those observed in rats in the present study.