1. Introduction
Intrathecal baclofen pumps are commonly used for the treatment of spasticity in a number of pathological processes, including cerebral palsy, stroke, traumatic brain injury, spinal cord injury, and multiple sclerosis. Various complications related to these drug delivery systems have been described in the literature, usually related to catheter malfunction or pump-catheter connection problems (1, 2). However, to our knowledge, baclofen pump migration into the peritoneal cavity has been reported in the literature only once (3). The authors describe here an interesting case of intraperitoneal baclofen pump migration in a 26-year-old male patient who originally had his baclofen pump placed in a subfascial location.
2. Case Presentation
A 26-year-old male patient with a history of spastic cerebral palsy, shunted hydrocephalus, and epilepsy was referred for his baclofen pump refill as well as increasing discomfort from spasticity. He was being treated with baclofen administered via an intrathecal baclofen pump that had been originally placed in subfascial position more than 20 years ago. His pump had been replaced twice previously. Specifically, he had undergone one previous end-of-service pump replacement and one revision to replace a fractured catheter, and the patient had not experienced any complications with any of his prior pump refills.
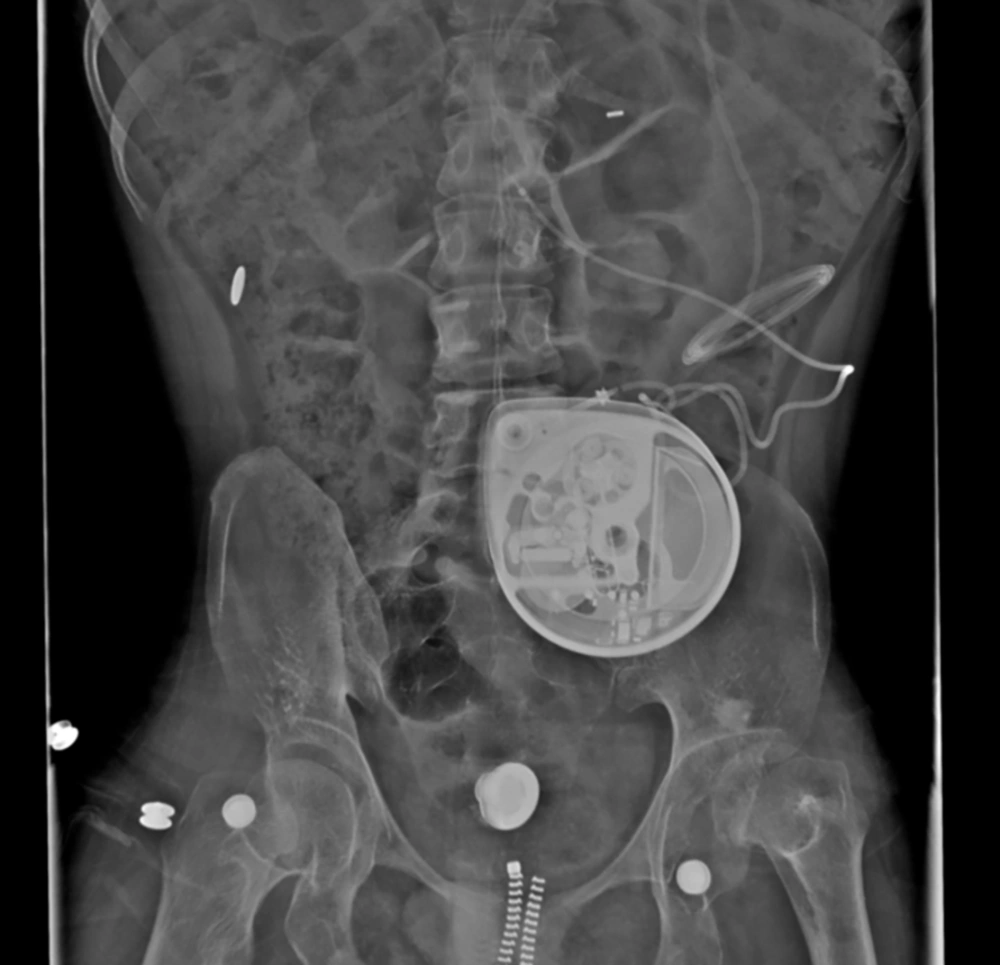
Because the patient’s baclofen pump could not be palpated in the created pocket and he continued to have signs of spasticity, an X-ray was performed, during his referral appointment at the clinic. The X-ray demonstrated a subcostal location of his baclofen pump, but it wasn’t clear from the X-ray whether the pump had migrated into the peritoneal cavity or not (Figure 1).
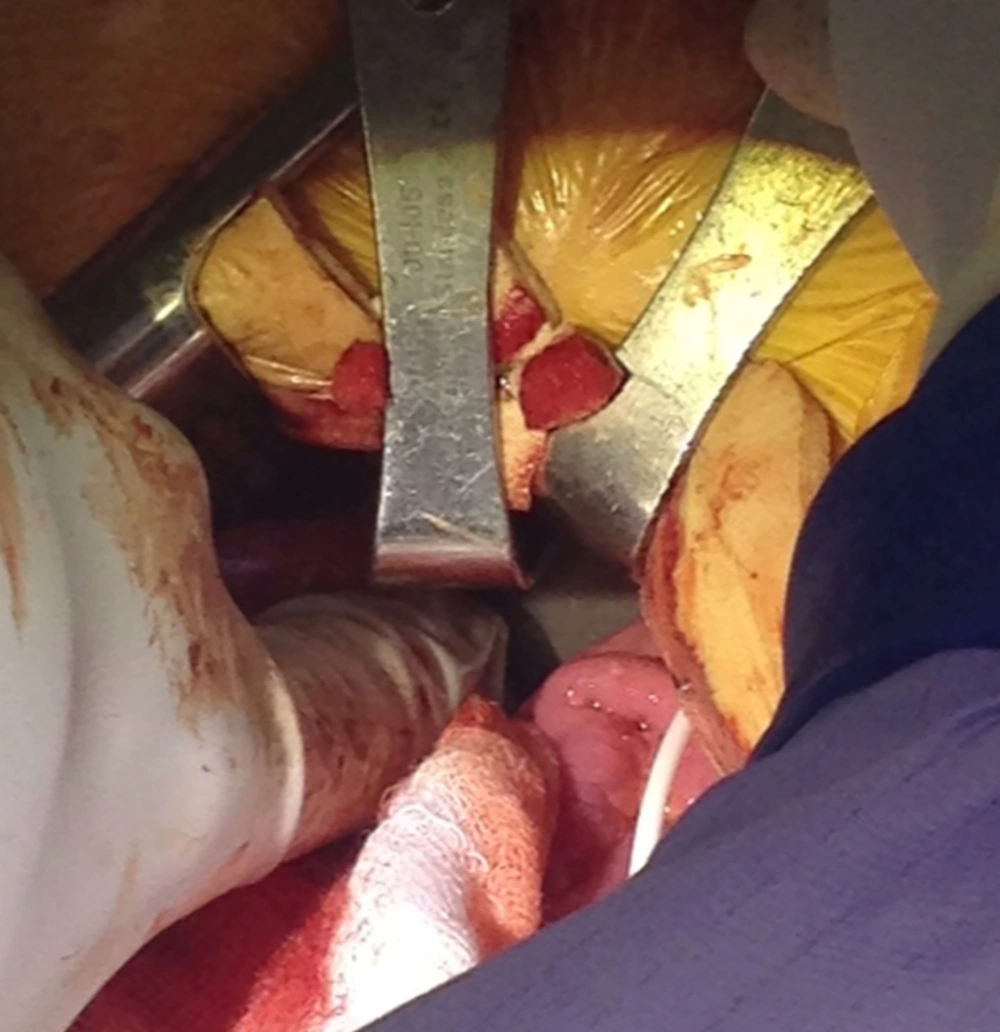
The patient was subsequently admitted and taken to the operating room for revision of his baclofen pump. Upon opening the subfascial pump pocket, we saw the pump catheter, but unexpectedly noticed that the pump had migrated. The dissection was extended along the catheter until the peritoneal cavity was entered where the 40-mL baclofen pump was found (Figure 2).
We removed his pump and inspected the bowel to ensure that no perforation had occurred. On further examination, we identified a fascial tear through which the baclofen pump had migrated. This defect in the fascia was in the right lower abdominal quadrant, on the posterior aspect of the subfascial pump pocket. For this reason, a smaller (20 mL) baclofen pump was filled and inserted instead subcutaneously.
Following the removal of his migrated baclofen pump and its replacement with a subcutaneous one, the patient was discharged home on postoperative day 7 only to return on postoperative day 9 with constipation and abdominal pain. Subsequently, he was readmitted and placed on a strict bowel regimen with milk of molasses enema treatments. As his diet was slowly advanced, he was monitored for any evidence of bowel distension. Once the patient was able to have normal bowel movements and tolerate oral food intake, he was discharged home on postoperative day 12. Additionally, on discharge it was noted that the patient would later need a proximal and distal revision of the ventriculoperitoneal shunt for his worsening hydrocephalus.
Due to progression of his hydrocephalus, the patient was then readmitted for a scheduled proximal and distal revision of his ventriculoperitoneal shunt. Although patient had significant adhesions, ventriculoperitoneal shunt revision was successfully performed. The patient was observed until postoperative day 7 to exclude the possibility of his ventriculoperitoneal shunt failure and sent home in good condition following this second procedure. However, on postoperative day 22, the patient began to develop evidence of shunt failure, including headaches and increasing ventriculomegaly. The patient was subsequently readmitted and returned to the operating room for a second revision of his ventriculoperitoneal shunt. Unfortunately, approximately one month later the patient passed away suddenly from what was believed to be sudden unexpected death in epilepsy. Of note, he was being managed with anti-epileptic drugs and intrathecal baclofen has no known effects on seizure exacerbation.
3. Discussion
Baclofen is a gamma-Aminobutyric acid (GABA) B receptor agonist that is commonly prescribed for the treatment of spasticity (1). Activation of GABAB receptors leads to inhibition of spinal reflexes, thereby decreasing spasticity. Oral baclofen is an effective treatment for spasticity as the medication readily crosses the blood brain barrier. However, side effects with oral baclofen therapy are common and may include fatigue and sedation. For this reason, intrathecal baclofen is preferred as a targeted therapy for patients suffering from spasticity (1, 4).
Two common techniques for intrathecal baclofen pump insertion include subcutaneous and subfascial pump placement. During subcutaneous placement, a small pocket is formed in the subcutaneous layer of the abdominal wall and the intrathecal catheter is tunneled to this pocket, where it is connected to the pump. Alternatively, another surgical technique is to place a baclofen pump subfascially, but the pump pocket is formed in the abdominal wall underneath the anterior rectus abdominus and the external oblique fascia (5, 6).
When compared to subcutaneous pump placement, subfascial pump placement is believed to reduce infection rates, especially in the pediatric population (5, 7). This is important to consider since higher infection rates overall have been demonstrated in children undergoing baclofen pump placement when compared with adults (8). For these reasons, baclofen pumps are frequently placed in a subfascial manner especially in the pediatric population. Additionally, subfascially placed pumps also tend to be more cosmetically appealing. Although preferred for all these reasons, subfascially placed pumps frequently require fluoroscopy for refills. They can also have serious complications such as pump migration into the peritoneal cavity, as described in this case report.
The authors here describe an interesting case of baclofen pump migration into the peritoneal cavity of a 26-year-old male patient who subsequently died from an unexpected death in epilepsy. The patient’s pump could not be palpated on the exam in the created pump pocket and he was experiencing increasing discomfort from spasticity. Of note, if an attempt to refill his pump had been made, a direct perforation of the bowel might have occurred since surgery later revealed that his pump had migrated into the peritoneal cavity through a fascial defect. It is essential to recognize that if there is any uncertainty during the evaluation for a patient’s pump refill, the pump should be imaged to confirm its current location and ideally compared to previous images. If there is still uncertainty, surgical exploration remains essential as in this case.
Our literature search found only one previously reported case of intrathecal baclofen pump migration into the peritoneal cavity. The authors there hypothesized that the migration of the pump was related to its placement below the linea semilunaris, leaving only a weak fascial layer formed by the fascia transversalis between the pump and the peritoneal cavity (3). In our patient, the subfascially placed baclofen pump migrated into the peritoneal cavity in a similar manner. During the exploratory laparotomy performed on our patient to retrieve the pump, an obvious fascial defect was also identified at the inferior portion of the fascia transversalis just above the inguinal ligament through which the pump had migrated. This defect in the fascia was in the right lower abdominal quadrant, on the posterior aspect of the subfascial pump pocket. However, the underlying mechanism behind pump migration into the peritoneal cavity remains to be elucidated.
Intrathecal baclofen pumps are valuable treatment options for those with spasticity from cerebral palsy, stroke, traumatic brain injury, spinal cord injury, and multiple sclerosis. Although subfascial pump placement is generally preferred over subcutaneous pump placement due to lower infection rate, rare complications can occur such as pump migration with this approach. Whether this is related to initial technique of pump insertion below linea semilunaris or not remains to be elucidated in the future. Nonetheless, we advise neurosurgeons to maintain a low level of threshold with regards to confirming the location of a baclofen pump with imaging and surgical exploration if its relocation is suspected in order to avoid detrimental outcomes such as bowel perforation.