1. Background
In endotracheal intubation using a laryngoscope, the sympathetic nervous system is stimulated to increase the plasma catecholamine concentration and induce complications such as tachycardia, hypertension, and arrhythmia (1, 2). These symptoms sometimes cause myocardial ischemia, left ventricular failure, or cerebral hemorrhage (3, 4). The adjunctive use of opioids, a β-adrenergic blocker, a vasodilator, and a calcium-channel blocker to inhibit these cardiovascular responses has been studied and reported (2, 4-7). Opioids neutralize the responses of the sympathetic nervous system and thus are widely used in laryngoscopic endotracheal intubation to inhibit cardiovascular responses (8-10). Their efficacy has been reported to be proportional to their dosage (11).
In double-lumen endobronchial intubation for one-lung ventilation during surgery, the tube has a larger diameter than that of the tube used in regular endotracheal intubation and must be inserted into the bronchus. Accordingly, the carina and the inner wall of the trachea are stimulated to induce more severe cardiovascular responses than in regular intubation (12). Moreover, patients who require one-lung ventilation often have cardiovascular diseases and therefore require additional attention.
Sufentanil is a quasi-compound of fentanyl that has a shorter onset time, a shorter duration of effectiveness, and potency 5 - 10 times greater than that of fentanyl, it causes less respiratory deterioration immediately after surgery than fentanyl does, and it is more effective in attenuating the cardiovascular intubation response than fentanyl (13). Thus, it has been widely used for anesthesia induction and maintenance (14-16). Nevertheless, no study on the appropriate dosage of sufentanil for double-lumen endobronchial intubation has been reported yet. Therefore, we conducted a randomized, double-blind study in which we administered various doses of sufentanil and observed the anesthetic depths and hemodynamic responses for each to determine an appropriate dosage for the attenuation of cardiovascular responses without adverse effects such as hypotension and bradyarrhythmia.
2. Objectives
The aim of this study was to determine the effective bolus dose of sufentanil to attenuate hemodynamic changes in response to double-lumen endobronchial intubation without adverse effects such as hypotension and bradyarrhythmia.
3. Patients and Methods
After we obtained institutional review board (Keimyung University, Dongsan Medical Center IRB, ref: 2013 - 03 - 014) approval of our study, the participants were informed of its purpose and asked to sign consent forms. The 72 subjects, aged 18 - 65 years, were supposed to have undergone elective surgical procedures that require one-lung ventilation. Their American Society of Anesthesiologists physical status was classified as 1 or 2. Patients with a history of cardiovascular diseases, renal dysfunction, intake of antihypertensive agents, upper airway abnormalities, and anticipated difficult intubation were excluded from the study.
The subjects were randomly assigned to one of four groups (18 members per group): the normal saline (NS) group (the control group), the 0.1 mcg/kg sufentanil (S0.1) group, the 0.2 mcg/kg sufentanil (S0.2) group, and the 0.3 mcg/kg sufentanil (S0.3) group. The assignment process entailed random index card sampling before patients entered the operating room; a resident not associated with the study issued to each patient a sealed index card marked with a group name and a number.
All subjects fasted for at least 8 hours before they underwent surgery. They were given 7.5 mg of midazolam orally as premedication the night before surgery, and then were given 0.2 mg of glycopyrrolate and 2 - 2.5 mg of midazolam by intramuscular injection 1 hour before the surgery. Upon their arrival at the operating room, an electrocardiography, a noninvasive automatic blood pressure monitor, and a pulse oximeter (Tram-rac 4A, GE Medical Systems, Milwaukee, WI, USA) were attached to the subjects to monitor their vital signs. The depth of the anesthesia was monitored using bispectral index scale (BIS) monitoring (BIS VISTA monitoring system, Aspect Medical Systems Inc., Norwood, MA, USA). After the subjects were preoxygenated with 100% oxygen, they were injected over a 30 second interval with a randomly selected sufentanil (Sufental INJ, BC world Pharm Co., Ltd., Korea) dose group. Each experimental agent was mixed with NS to make its total volume 5 mL by a nurse not associated with the study. One minute after the administration of the experimental agent, 2 mg/kg of propofol was injected over 30 seconds to induce loss of consciousness, and 0.9 mg/kg of rocuronium was administered. Two minutes after rocuronium administration, double-lumen endobronchial intubation (Broncho-Cath, Mallinckrodt Medical, Athlone, Ireland) was performed using a MacIntosh laryngoscope by one expert anesthesiologist. An anesthesiologist performed simple blind intubation into the left bronchus without knowledge of the dose of the experimental agent or of readings from the hemodynamic monitor, with sevoflurane at 1.5 - 3 vol% in 1.5 l/minutes air and O2, a tidal volume of 8 - 10 mL/kg, and a respiratory rate of 8 - 12 times per minute to maintain an end tidal carbon dioxide pressure of 35 ± 5 mmHg. Time to intubation (time from laryngoscopic manipulation to expansion of the tube cuff balloon) was recorded. The cases of failure of intubation on first attempt were excluded from data collection.
When bradycardia developed with a heart rate (HR) of < 50 beats per minute, 0.5 mg of atropine was injected. When systolic blood pressure (SBP) was ≤ 80 mmHg two or more consecutive times, 8 mg of ephedrine was injected. When hypertension and tachycardia developed two or more times, 1 mg of nicardipine hydrochloride or 10 - 20 mg of esmolol was injected.
Bispectral index scale values and hemodynamic changes, such as in SBP, diastolic blood pressure (DPB), mean arterial pressure (MAP), and HR, were measured and recorded before anesthesia induction (baseline), immediate pre-intubation, immediate post-intubation, and every minute after intubation for 5 minutes.
When a bronchoscopic confirmation or additional procedure was needed after intubation, it was performed after hemodynamic changes were measured for 5 minutes after intubation to ensure the same study conditions.
A power analysis based on previous data (17) indicated that at least 18 patients in each group would be required to show a difference between groups of 20 mmHg in the MAP during intubation (α = 0.05; β = 0.2).
The data were statistically analyzed with SPSS software (version 22.0; IBM, Armonk, NY, USA). The Kolmogorov Smirnov test was done for checking normal distribution. The demographic data for all groups were compared using one-way analysis of variance (ANOVA), intragroup differences in hemodynamic data over time were measured using repeated-measures ANOVA, and intergroup differences in hemodynamic data at each time point were compared using one-way ANOVA. The Tukey test was used for multiple comparisons. Quantitative data are expressed here as means ± SD. P <0.05 were considered statistically significant.
4. Results
No significant differences regarding age, sex, height, or weight among the groups were observed. In addition, there were no statistically significant differences before anesthesia induction regarding SBP, DBP, MAP, HR, BIS value, or time to intubation (Table 1).
| Variables | NS Group | S0.1 Group | S0.2 Group | S0.3 Group | P Value |
|---|---|---|---|---|---|
| Age, y | 44.3 (16.7) | 43.4 (18.8) | 44.4 (17.2) | 45.9 (17.2) | 0.980 |
| Gender | 0.770 | ||||
| Male | 15 | 14 | 13 | 14 | |
| Female | 3 | 4 | 5 | 4 | |
| Height, cm | 168.2 (7.8) | 170.4 (8.8) | 167.4 (7.3) | 168.5 (9.8) | 0.738 |
| Weight, kg | 65.1 (8.8) | 64.6 (8.9) | 60.0 (10.8) | 63.8 (12.8) | 0.444 |
| SBP, mmHg | 124.9 (13.7) | 122.6 (12.3) | 124.0 (18.9) | 126.4 (14.0) | 0.693 |
| DBP, mmHg | 72.9 (13.8) | 69.5 (13.2) | 71.6 (12.5) | 73.7 (10.9) | 0.768 |
| MAP, mmHg | 89.8 (12.1) | 89.2 (10.9) | 88.8 (13.6) | 90.7 (11.1) | 0.968 |
| HR, beats per minute | 73.3 (12.5) | 72.3 (12.4) | 76.4 (15.2) | 77.1 (17.1) | 0.701 |
| BIS value | 91.1 (4.7) | 91.1 (4.7) | 90.6 (5.1) | 90.7 (4.9) | 0.985 |
| Time to intubation, sec | 15.7 (7.5) | 16.9 (8.2) | 15.7 (5.9) | 16.4 (6.1) | 0.934 |
aValues are presented as means (SD), except for sex, which is presented as number of patients.
Abbreviations: BIS, bispectral index scale; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; NS, control group received normal saline; S0.1, group receiving 0.1 mcg/kg of sufentanil; S0.2, group receiving 0.2 mcg/kg of sufentanil; S0.3, group receiving 0.3 mcg/kg of sufentanil; SBP, systolic blood pressure.
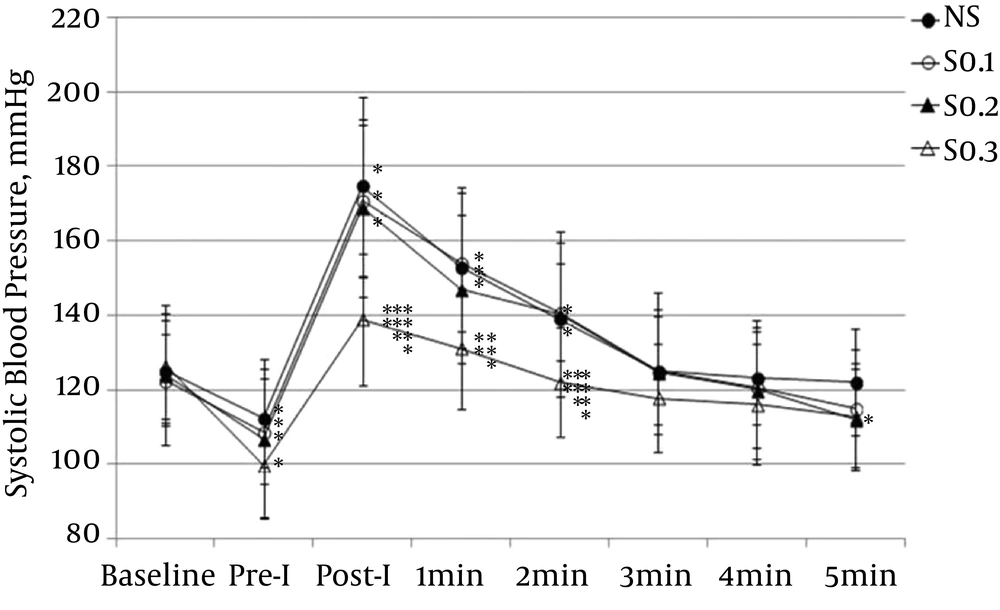
The SBP significantly decreased from baseline at immediate pre-intubation in all groups (P < 0.01), but not to the level of hypotension. At immediate post-intubation, the mean SBP significantly increased from baseline in all groups (P for NS < 0.001; P for S0.1 < 0.001; P for S0.2 < 0.001; P S0.3 < 0.05), but SBP of the S0.3 group was 138.9 ± 17.8 mmHg, which is within the normal blood pressure range. In the time point comparison of the SBP among groups, the S0.3 group had significantly lower values than did the NS, S0.1, and S0.2 groups at immediate post-intubation and 2 minutes after intubation (Figure 1).
Values are presented as means ± SD. NS: group receiving normal saline; Pre-I: preintubation; Post-I: post intubation; S0.1: group receiving 0.1 μg/kg of sufentanil; S0.2: group receiving 0.2 μg/kg of sufentanil; S0.3: group receiving 0.3 μg/kg of sufentanil. *: P < 0.05 compared with baseline; **: P < 0.05 compared with NS; ***: P < 0.05 compared with S0.1; ****: P < 0.05 compared with S0.2.
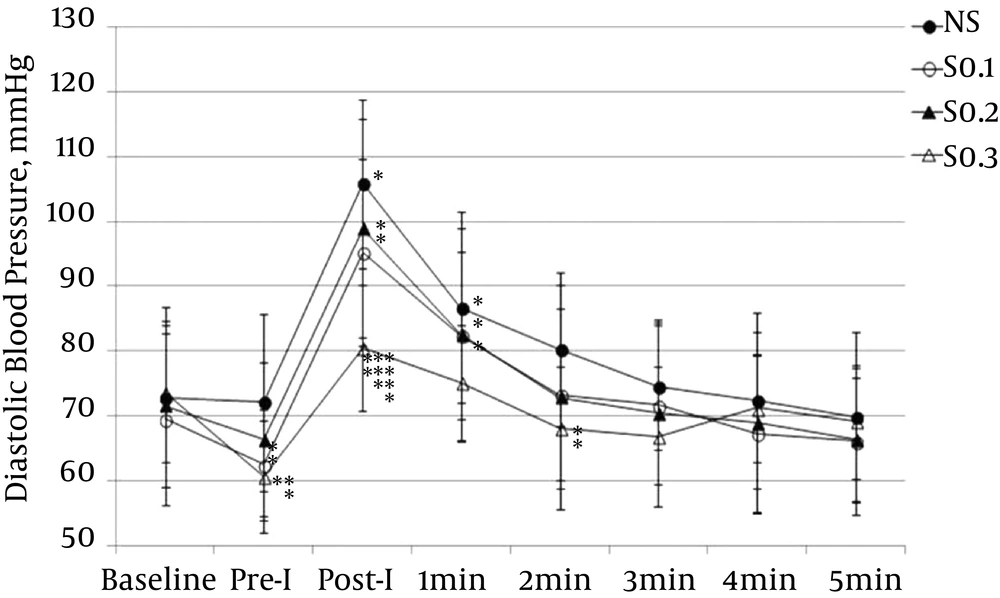
The DBP significantly increased from baseline at immediate post-intubation in the NS, S0.1, and S0.2 groups (P < 0.001). At immediate pre-intubation, the mean DBP of the S0.3 group significantly decreased, but not to the level of hypotension. In the time point comparison among groups at immediate post-intubation, the mean DBP of the S0.3 group was significantly lower than in the NS, S0.1, and S0.2 groups (Figure 2).
Values are presented as means ± SD. NS: group receiving normal saline; Pre-I: preintubation; Post-I: post intubation; S0.1: group receiving 0.1 μg/kg of sufentanil; S0.2: group receiving 0.2 μg/kg of sufentanil; S0.3: group receiving 0.3 μg/kg of sufentanil. *: P < 0.05 compared with baseline; **: P < 0.05 compared with NS; ***: P < 0.05 compared with S0.1; ****: P < 0.05 compared with S0.2.
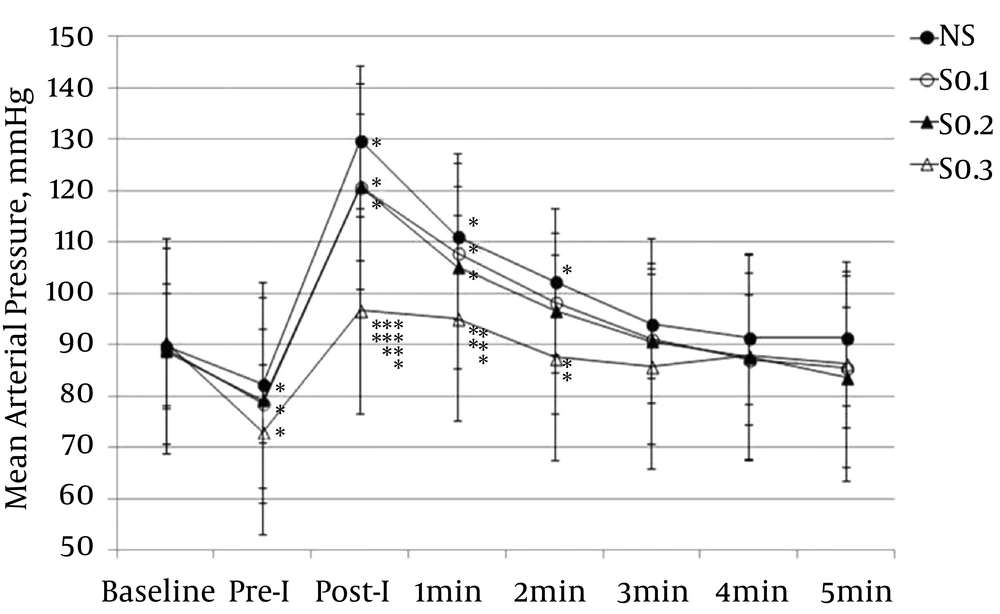
The MAP at immediate pre-intubation in the S0.1, S0.2, and S0.3 groups was significantly lower than at baseline (P < 0.001, P < 0.01, and P < 0.001, respectively), but not to the level of hypotension. At immediate post-intubation, the MAP in the NS, S0.1, and S0.2 groups significantly increased from baseline (P < 0.001) until 1 minute after intubation (P < 0.001). In the time point comparison among groups at immediate post-intubation, the MAP in the S0.3 group was significantly lower than in the NS, S0.1, and S0.2 groups, and the MAP at 1 minute after intubation was significantly lower in the S0.3 group than in the NS and S0.1 groups (Figure 3).
Values are presented as means ± SD. NS: group receiving normal saline; Pre-I: preintubation; Post-I: post intubation; S0.1: group receiving 0.1 μg/kg of sufentanil; S0.2: group receiving 0.2 μg/kg of sufentanil; S0.3: group receiving 0.3 μg/kg of sufentanil. *: P < 0.05 compared with baseline; **: P < 0.05 compared with NS; ***: P < 0.05 compared with S0.1; ****: P < 0.05 compared with S0.2.
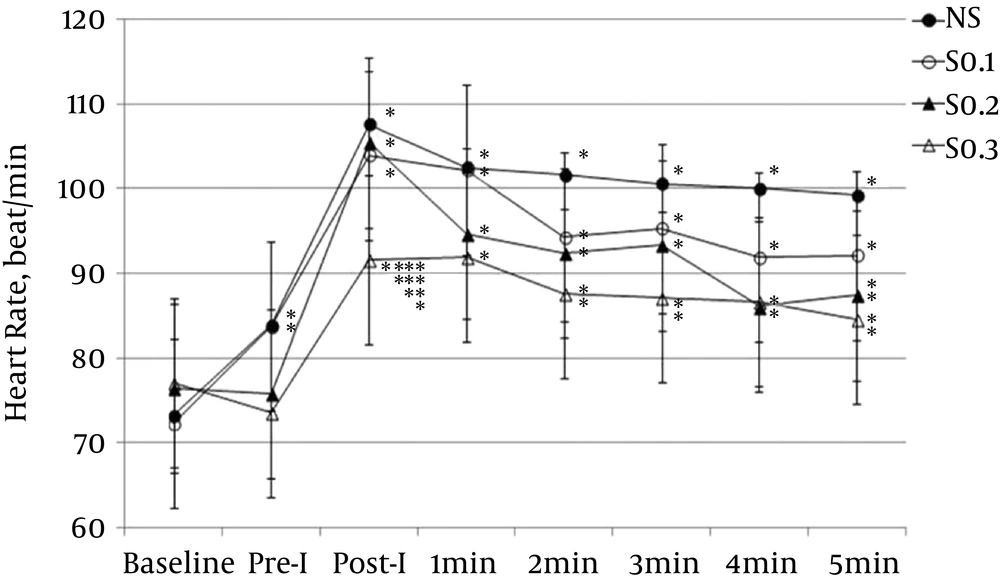
The HR at immediate post-intubation and 1 minute after intubation significantly increased from baseline in the NS, S0.1, S0.2, and S0.3 groups (P < 0.001). However, the mean HR in the S0.3 group was within normal range. In the time point comparison among groups at immediate post-intubation, the HR in the S0.3 group was significantly lower than in the NS, S0.1, and S0.2 groups (Figure 4).
Values are presented as mean ± SD. NS: group receiving normal saline; Pre-I: preintubation; Post-I: post intubation; S0.1: group received 0.1 μg/kg of sufentanil; S0.2: group received 0.2 μg/kg of sufentanil; S0.3: group received 0.3 μg/kg of sufentanil; *: P < 0.05 compared with baseline; **: P < 0.05 compared with NS; ***: P < 0.05 compared with S0.1; ****: P < 0.05 compared with S0.2.
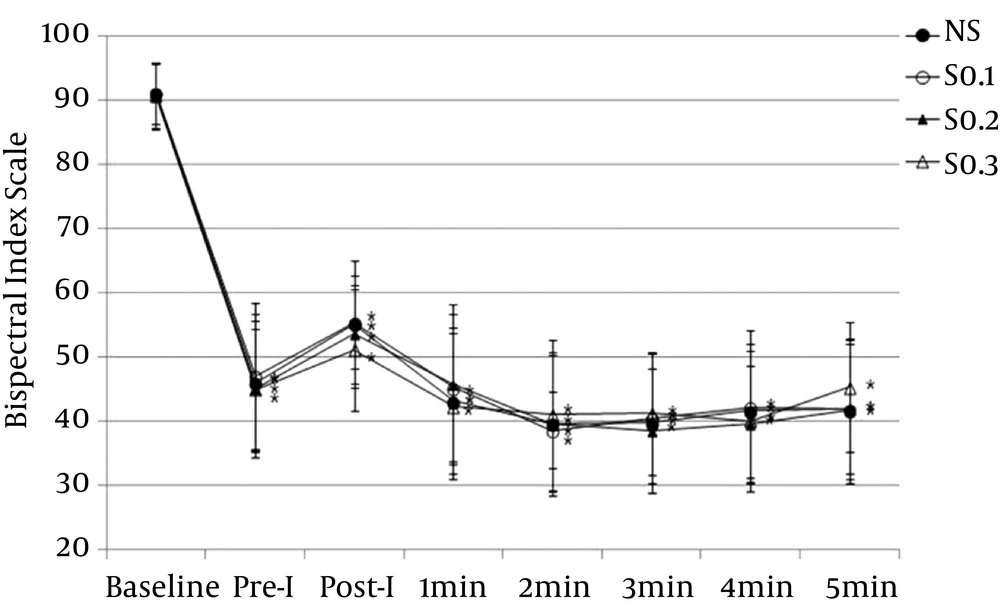
The BIS values for all groups significantly decreased from baseline after anesthesia induction, remaining in an appropriate range for anesthesia. No significant difference was observed among the groups in the time point comparison between groups (Figure 5).
Values are presented as mean ± SD. NS: group receiving normal saline; Pre-I: preintubation; Post-I: post intubation; S0.1: group receiving 0.1 μg/kg of sufentanil; S0.2: group receiving 0.2 μg/kg of sufentanil; S0.3: group receiving 0.3 μg/kg of sufentanil. *: P < 0.05 compared with baseline.
No patients needed ephedrine or atropine for hypotension or bradycardia during anesthesia induction, and there were no cases of ventilatory difficulty due to chest rigidity. The number of subjects who had two or more consecutive bouts of hypertension or tachycardia requiring the administration of esmolol or perdipine was 13 (72%) in the NS group, 12 (67%) in the S0.1 group, 4 (22%) in the S0.2 group, and 1 (5%) in the S0.3 group. There were no cases of failure of intubation on first attempt.
5. Discussion
One-lung ventilation, which involves double-lumen endobronchial intubation, is required in various surgeries, including comparatively simple pneumothorax surgery, lobectomy, aneurysm of the thoracic aorta, esophageal surgery, and pulmonary embolectomy. Few studies on effectively inhibiting cardiovascular responses have been reported. In particular, no reports of studies on the appropriate dosage of sufentanil have been published yet. Thus, our randomized, double-blinded study investigated the use of propofol combined with sufentanil in various doses in double-lumen endobronchial intubation for one-lung ventilation. The hemodynamic responses at each dose were analyzed, and the appropriate dose of sufentanil, which could inhibit cardiovascular responses without adverse effects such as hypotension and bradycardia, was investigated.
Casati et al. reported that cardiovascular responses were inhibited by a bolus injection of 0.1 mcg/kg sufentanil followed by continuous injection of sufentanil at 0.01 mcg/kg/min during tracheal intubation (18). In addition, Kay et al. reported that 0.5 mcg/kg and 1.0 mcg/kg of sufentanil were effective but that with 0.5 mcg/kg, a significantly lower blood pressure and HR than at baseline were observed for 15 minutes after intubation (19). With 1.0 mcg/kg of sufentanil, spontaneous breathing was not recovered for 15 minutes after intubation, which means there was a strong dose-related adverse effect.
In a study on anesthesia induction using propofol that targeted children, the cardiovascular intubation responses were not appropriately depressed with the administration of 0.2 mcg/kg of sufentanil, but they were fully depressed with 0.3 mcg/kg (14, 15). The difference in the appropriate dosages for adults and children might have been due to the pharmacokinetic difference, which refers to the 1.5 times larger volume of distribution for children in a steady state than for adults (20). Considering those study results, the targets of our study (adults), and the type of intubation (not regular tracheal intubation but double-lumen endobronchial intubation), we chose experimental doses of sufentanil of 0.1 mcg/kg, 0.2 mcg/kg, and 0.3 mcg/kg. In addition, the reason for the bolus injection of sufentanil without continuous injection was that the time spent for intubation after sufentanil administration was no longer than 6 minutes, and the hemodynamic changes caused by intubation lasted only 2 - 5 minutes, so once the dose and timing of administration were appropriately selected, the single bolus injection was effective enough. In addition, the experimental error caused by continuous injection could be reduced, and even in the case of a short duration of surgery, recovery of spontaneous breathing might not be affected. When the duration of surgery was long or when an additional dose of sufentanil was necessary, bolus or continuous injection was conducted 5 minutes after intubation.
Our findings confirmed that the bolus sufentanil injection dose necessary to effectively inhibit the cardiovascular responses caused by double-lumen endobronchial intubation in anesthesia induction using intravenous anesthetics such as propofol and a neuromuscular blocking drug was 0.3 mcg/kg. Other than the S0.3 group, the NS, S0.1, and S0.2 groups showed statistically significant hemodynamic changes at immediate post-intubation compared with baseline, and the effect lasted until 1 - 2 minutes after intubation. In the S0.3 group, a higher SBP and HR than at baseline were observed at immediate post-intubation, but the values were within normal ranges. The time point comparison among groups showed that the levels of all cardiovascular responses (SBP, DBP, MAP, and HR) of the S0.3 group at immediate post-intubation were significantly lower than for the NS, S0.1, and S0.2 groups.
The S0.1 and S0.2 groups showed abrupt increases in hemodynamic changes at immediate post-intubation and until 1 or 2 minutes after intubation, so their doses were not considered appropriate for inhibiting cardiovascular responses in double-lumen endobronchial intubation. Only the S0.3 group showed clinically satisfactory inhibition. Of course, an increased injection dose of sufentanil could have been more effective for the inhibition of cardiovascular responses and could have improved the intubation environment, but the risk of adverse effects such as hypotension and bradycardia could have increased as well.
Regarding the timing of the administration of the drugs in our study, sufentanil and propofol were administered 5 minutes and 3 minutes before intubation, respectively, considering that the peak time of sufentanil in adults was 5 - 6 minutes (21) and the time spent to reach the peak effect site concentration of propofol was 3 minutes (22). In addition, 0.9 mg/kg of rocuronium, a neuromuscular blocking drug, was administered immediately after propofol injection and loss of consciousness to secure a sufficient intubation environment.
Unlike other opioids, the separate use of sufentanil is known to induce less cardiovascular instability. When it is used in combination with other agents or excessively, however, abrupt hypotension and bradycardia may develop (23). These cardiovascular responses are thought to be secondary responses caused by opioid receptors that affect the central nervous system (24). The most common adverse effects of the intravenous administration of a medium or high dose of sufentanil include hypotension, chest wall rigidity, and bradycardia, and their incidence rates are 6%, 2.9%, and 3.4%, respectively (23). In our study, the combination of a small dose sufentanil and propofol did not result in hypotension or bradyarrhythmia that required treatment, and symptoms such as chest rigidity and violent coughing were not observed. We presume that these complications did not develop because the sufentanil dose was not very high and was injected slowly over 30 seconds.
Two of the factors in choosing the dose of the opioid adjunctively used for endotracheal intubation are the type and dose of the main anesthetic agent. On the basis of the outcomes of previous studies on the effects of sufentanil on the inhibition of cardiovascular responses, we chose to use propofol as an anesthesia induction agent. Because there was no significant difference in the BIS values that were continuously measured before and after anesthesia induction and in the concentration of sevoflurane that had been inhaled since immediately after intubation, we attribute the difference in the hemodynamic changes immediately after intubation and up to 1 - 5 minutes after intubation to the difference in sufentanil dose.
In conclusion, we found that in laryngoscopic double-lumen endobronchial intubation using propofol for anesthesia induction, 0.3 mcg/kg was the optimal dose of sufentanil for the depression of cardiovascular responses with minimal adverse effects such as hypotension and bradycardia.