Dear Editor,
We report a case of 15.4 cm abdominal aorta aneurysm, which underwent an emergent open repair. Our patient is a 64-year-old female, with past medical history of Type 2 diabetes, hypertension and hyperlipidemia who presented to the emergency department with a two-day history of constant progressively worsening abdominal pain. Physical exam was remarkable for a distended abdomen with pulsatile tender mass in the right peri-umbilical region.
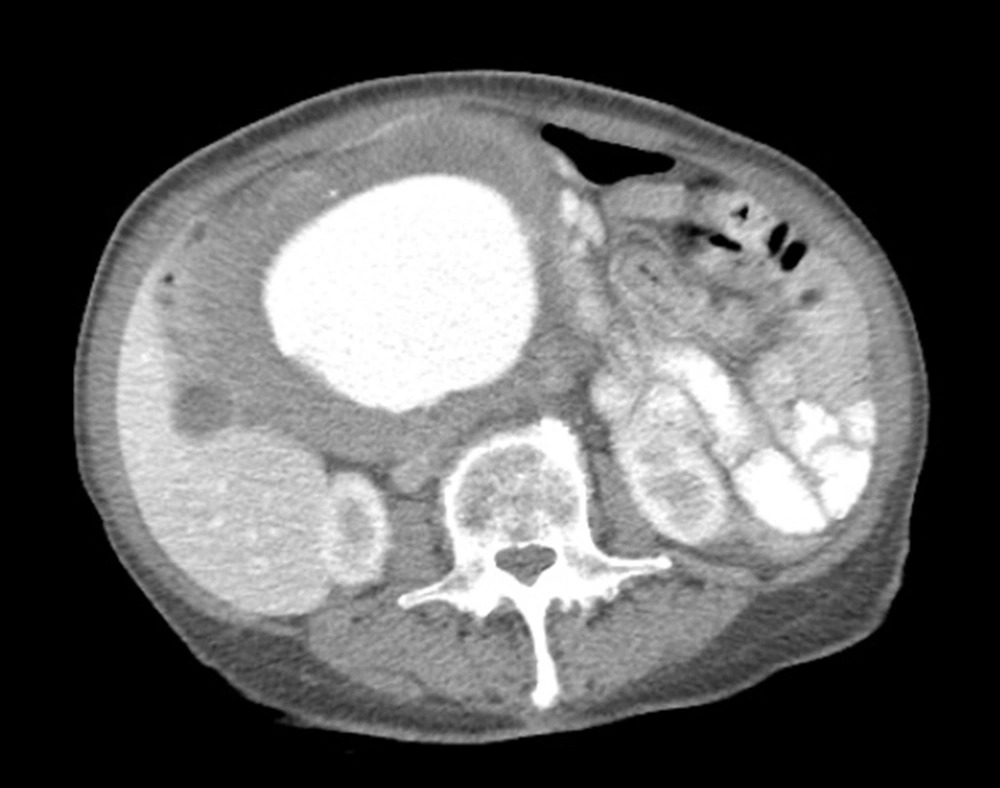
CT abdomen and pelvis with contrast showed a fusiform tortuous abdominal aortic aneurysm immediately distal to the renal artery ostia at the level of the L1 vertebrae measuring 1.8 cm with extension into the bilateral common iliac arteries at the level of the bifurcation with sparing of the celiac and SMA (Figure 1). The patient was relatively healthy, but had stopped her hypertensive medications for the last year due to monetary issues and had no cardiac workup upon admission. The patient’s transesophageal echocardiography (TEE) showed LVEF of 60% - 65%, PASP 35 - 40 mmHg, mild MR and TR with no wall motion abnormalities. Cardiology was consulted for risk stratification where she was declared to be low risk for a high-risk procedure. Patient was observed overnight in the intensive care unit and surgery was planned for the following morning.
In the operating room, standard ASA monitors were applied. A left axillary arterial line for invasive monitoring of blood pressure and a right internal jugular cordis was inserted under local anesthesia. Gentle anesthetic induction was achieved with intravenous propofol, fentanyl and rocuronium. Tracheal intubation was uneventful with tight control of systolic blood pressure, as there was a high risk of rupture due to size. Baseline echocardiography exam was obtained via TEE. Estimated EF was 50% - 55%. ACT’s were monitored throughout the procedure.
Open surgical repair was carried out with a midline incision in three stages using a Hemashield bifurcated iliac graft. The third and fourth portions of the duodenum were contiguous with the aneurysm. Firstly, after systemic heparinization the external iliac arteries were clamped followed by infra-renal aortic clamping, dissection and evacuation of thrombus with end-to-end anastomosis of the graft to the infra-renal aorta. A clamp was applied to the graft itself followed by slow release of aortic clamp to verify suture line haemostasis. The second and third stages of repair involved clamping the internal iliac arteries, dissection and end-to-end anastomosis the graft to distal common iliac arteries.
Fluid was restricted prior to cross clamping. As intraoperative hypotension has been associated with post-operative acute kidney injury we assured a minimum urine output of 0.5 mls/kg/hour (1). Systolic BP maintained in the low-normal range of 100 - 125 mmHg. Initial fluid boluses were based upon TEE findings, CVP trend, arterial blood pressure and urine output measurements. On application of the infra-renal cross clamp, an increase in end diastolic volume was appreciated on TEE even though minimal alteration in the hemodynamics was observed. Trans-gastric short axis mid papillary view was used to monitor volume status with an intermitted nitroglycerin drip utilized to control preload with dose ranging from 30 - 50 mcg/min. Minute ventilation was increased prior to clamp removal. No hypotension was observed post clamp removal possibly due to the staged release. Total cross clamp time was 2 hours and 7 minutes. Total UOP was 390 mls of which 100 mls was in the 1 hour immediately post reperfusion. EBL was 3,500 mls. 7 units PRBC and 3 FFP were transfused in addition to 4.5 liters of isolyte to keep Hb above 8.
Upon completion of the surgery, patient was transferred to the surgical ICU intubated. She was extubated on POD #1 and discharged on POD #8. Her serum creatinine on the day of discharge was 1.2 (0.86 baseline). This may be attributed to the decrease in renal blood flow that is possible even with infra-renal clamping.
Due to the size, tortuosity of the iliac arteries and adhesion to intra-abdominal structures an endovascular approach was not a feasible option. Tight blood pressure control was very pertinent in this particular case. At 15.4 cm we believe the AAA cited here is one of the largest to date (2-4).