1. Background
Non-surgical chronic low back pain is among the most common, debilitating and expensive conditions seen in primary care and pain management practices (1, 2). Common diagnoses include lumbar spinal stenosis, lumbar radiculopathy, post laminectomy pain and nonspecific low back pain. Treatment options include injection into the caudal epidural space of agents such as anti-inflammatories, analgesics and anesthetics.
Fluoroscopic and ultrasound-guided caudal epidural injection typically use a cephalad needle angulation. The needle is advanced slightly through the sacral cornua (3) using a 22-gauge spinal needle (3). Aspiration is performed, with repositioning if blood return is noted. If a trial small volume dye injection confirms a positive epidurogram, and no systemic reaction to lidocaine is observed, the full injectate volume is delivered. Renfrew et al. advocated for use of fluoroscopic guidance after reporting that, despite confirmation of needle placement within the spinal canal and no blood return, the injection resulted in venous placement in 29/316 procedures (9. 2%) (4) Subsequent reports have generally demonstrated lower intravascular injection rates, but the procedure, with a cephalad needle direction; can result in vascular entry despite ideal needle positioning (5).
One of the co-authors (HR) developed an alternative caudal epidural injection technique, described as a vertical small-needle technique because needle penetration is vertical to the skin surface; needle penetration is at or below the approximate sacral corneal level. This approach relies on the anatomic location of the sacral epidural space which extends caudally to the sacrococcygeal junction and is filled with fluid, fat, and loose areolar connective tissue that allows the spread of the injected solution in a cephalad direction (3). Potential advantages compared to the traditional approach include less risk of intravascular injection due to vertical needle orientation and small needle gauge and an increased rate of epidural space entry with first needle placement due to decreased needle wander. However, this technique has not been formally assessed.
2. Objectives
We conducted an open-label prospective study to test the hypotheses that vertical small-needle caudal epidural injection without fluoroscopic or ultrasonographic guidance will achieve at least 80% first attempt needle placement in the epidural space as assessed by epidurogram and will not result in intravascular injection.
3. Patients and Methods
The study protocol conformed to the ethical guidelines of the 1975 declaration of Helsinki and was approved by the Western institutional review board.
3.1. Eligibility Criteria and Participant Recruitment
Adults 19 to 75 years of age were enrolled and received epidural injections from February, 2013, to November, 2014; each was a participant in a randomized and blinded clinical trial designed to assess the analgesic (6) (not anesthetic) (7) effect of caudal epidural dextrose injection compared with saline control injection. Those that received control saline injection thereafter received active dextrose injection. Participants in both dextrose and saline study arms consented to receive their caudal injections using the vertical small-needle injection technique described in this study. They were recruited from the communities of Hilo and Honolulu, Hawaii. Inclusion criteria included non-surgical chronic low back pain (more than 6 months) with gluteal and/or leg pain. Eligible participants had current pain levels 5/10 or higher on a 0 - 10 numerical rating scale and failure of at least one non-injection treatment modality including physical therapy, chiropractic manipulation, exercises, drug therapy or relative rest. Diagnoses accepted included lumbar spinal stenosis, lumbar radiculopathy, post laminectomy pain, peripheral neuropathy and nonspecific low back pain. Exclusion criteria included pregnancy, recent changes in opioid use, unstable psychiatric disorder, medical instability, anticoagulation therapy or local infection.
3.2. Outcome Measures
The primary outcome measures were the percentage of positive epidurography demonstrating successful needle placement and the percentage of intravascular injection, both with first needle positioning and with repositioning as needed.
The epidurogram patterns were analyzed real-time by a sports arts and musculoskeletal medicine fellowship certified physiatrist who performs fluoroscopically-guided caudal epidurals regularly, and who performed the caudal epidural injections in this study. At each follow-up visit patients were asked to provide qualitative feedback about any side effects or adverse events of the previous injection and all such comments were recorded.
3.3. Intervention
Determination of the approximate sacral hiatus level and point of midline needle entry utilized a commonly taught manual approach in which the tip of the middle finger is placed at the tip of the coccyx and, depending upon the hand size, the sacral cornual level is estimated as just proximal to, at, or just distal to the crease of the PIP joint (8). A 25 gauge 37 mm hypodermic needle was attached to a 5 mL syringe, filled with iopamidol, and advanced through the skin at an approximate 90 degree angle. As the sacrococcygeal ligament was penetrated, a “pop and drop” was felt with the needle gently coming to rest in light contact with the posterior sacrum. Gentle aspiration was then performed to identify blood or CSF. If positive for CSF or blood, the participant was rescheduled. Upon negative aspiration, 2 - 3 mL of iopamidol was instilled and the filling pattern was observed under fluoroscopy in the lateral view. A regular, linear and stable pattern was considered a positive epidurogram. A regular pattern that was nonlinear or disappeared with observation was documented as an intravascular pattern and the participant was rescheduled. An extradural pattern was documented as an irregular or “cotton ball” appearance. If an intravascular or extradural pattern was seen, the needle was slightly redirected using primarily a skin slide technique either left, right, or up to 10 degrees cephalad, until a positive epidurogram pattern was seen.
3.4. Analysis
Data was analyzed using PASW 18 (Predictive Analytics Software 18. 0. 0, IBM). Descriptive statistics were utilized to determine the percentage of positive epidurography and intravascular injection at first needle placement and after repositioning as needed.
4. Results
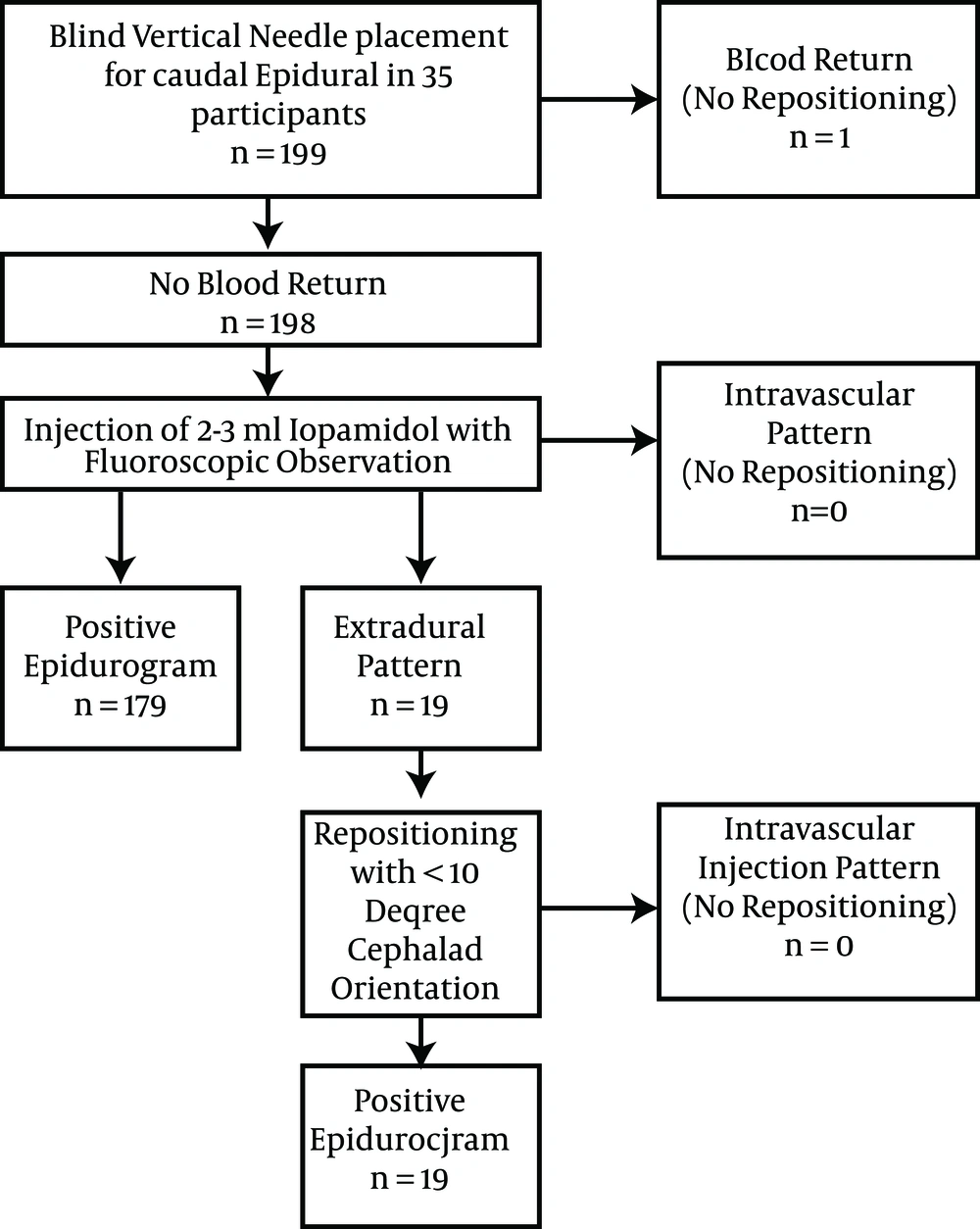
The recruitment and participation scheme is given in (Figure 1). Thirty-five participants, eleven (31%) female, were enrolled. Participants were 54 ± 11 years old, weighed 89 ± 22 kg, and had a BMI of 30 ± 7.3 Kg/m2. One hundred ninety nine vertical small-needle caudal epidural injections were performed in these 35 participants, 182 with 5% dextrose in water and 17 with normal saline. Blind needle placement resulted in a positive epidurogram in 179/199 (90%) at first needle placement, an extradural pattern in 19/199 and blood return in 1/199 with rescheduling of that participant. Those participants with an extradural pattern had positive epidurography with minimal repositioning. No intravascular injection patterns were observed, either upon initial contrast injection or after repositioning. No vasovagal events occurred, and prolonged discomfort after the procedure was not reported at follow-up.
5. Discussion
5.1. Main Finding
In 199 consecutive vertical small-needle caudal epidural injections performed without imaging guidance, the epidural space was successfully entered 90% of the time at first needle placement, as assessed by contrast epidurography, and no intravascular injection pattern was demonstrable at first entry or with repositioning. We postulate that the lack of intravascular injection in our study is due to the vertical needle direction and small needle gauge, limiting the potential for flow into a vessel.
5.2. Literature Discussion
The positive epidurography success rate of 90% with manual (‘blind’) vertical needle placement compares to a typical blind needle success rate of epidurography of 60% - 75% using a cephalad needle direction and advancement through the cornua, even after needle repositioning (4, 9-12). The success rate of 99.5% (198/199) with repositioning (with one aborted due to blood return) compares favorably to the highest success rates reported in fluoroscopic guided literature (5, 13). Our rate of 90% positive epidurography at first needle placement may be attributable to less needle wander due to a short distance of vertical needle advancement.
The positive blood return rate at first blind needle placement was 1/199 (.05%) in the current study. This success rate may exceed that of fluoroscopically guided injections. With use of ultrasound guidance to place the needle with a cephalad orientation through the cornua 9/29 (31%) had initial blood return in one study (14) and at least 5/25 (25%) in another (15). However, although aspiration for blood likely reduces the incidence of intravascular drug injection, it is not sufficient to prevent intravascular injection (5). The rate of intravascular injection ranges from 2.5% to 9% for fluoroscopically-guided caudal epidural injection, (5, 9, 10) compared to 0% in the current study.
The frequency of anatomic irregularities resulting in failed caudal epidural with fluoroscopically-guided method varies from 1% - 3% when documented (4, 11, 16, 17). Reasons for epidural failure are primarily due to unusual anatomy such as a narrow sacral canal interfering with needle advancement beyond the cornua, particularly with a spinal canal height of 1.5 mm or less (18). However, in the absence of a rare sacral canal agenesis, fluid placed at or below cornual level, such as in this method, can still flow cephalad through a narrow sacral canal. Screening for congenital abnormalities is not recommended prior to the vertical short-needle caudal epidural approach, and Doppler observation of the hiatal region can quickly document sacral canal agenesis.
Two studies have suggested that caudal epidural injection may be more safely performed with Doppler monitor than with fluoroscopic guidance through decreasing the risk of intravascular injection, one by use of Doppler flow confirmation of an epidural pattern (5) and one by injecting immediately after penetrating the sacrococcygeal membrane to avoid contact with the venous plexus (15). This study adds another method to avoid intravascular injection, by inserting the needle at a 90 degree angle to the plexus. Ultimately ultrasound-guided epidural injection appears to have important advantages, and the use of Doppler confirmation of an epidural flow pattern in combination with the vertical short-needle caudal epidural technique may lead to further improvements in safety and in patient comfort. If the needle is not advanced beyond the cornua with either this vertical technique or with the technique using injection immediately after sacrococcygeal ligament penetration, the tip of the needle is easily observable for a Doppler flow pattern check (5). A study utilizing Doppler flow for confirmation of an epidural injection pattern, and comparing the vertical short needle caudal epidural technique to a cephalad needle approach, would provide further information on the relative merit of each approach.
Short needle advancement distance in vertical approach, small needle gauge, and minimal needle repositioning may contribute to patient tolerance of this technique. Although comfort-related data was not rigorously collected in this study, no vasovagal reactions occurred and prolonged post-injection sacral area soreness was not reported. The caudal epidural literature does not routinely report the number of vasovagal reactions as complications. Park indicated post-procedure vasovagal reactions in 2/60 with ultrasound-directed and 3/60 with fluoroscopically-guided caudal epidural injection, (5) although Manchikanti et al.’s (19) data suggests a low incidence of vasovagal reactions.
5.3. Limitations
The main limitation of this study is that it is a single arm study. It does not compare the effectiveness of the vertical approach to that of another technique. However, effectiveness appears to compare well to published success rates of other techniques. This was a single physician study. Palpation skills for fingertip-guided injection would be operator-specific. Repetition of this study comparing the success rate of multiple operators is necessary to more completely evaluate the vertical small-needle caudal epidural technique. In addition, this study included only two participants whose anatomy may present technical challenges. (BMI > 40) However, ultrasound can be utilized to aid in determining anatomic variations and, in these authors’ experience, a 25 gauge 37 mm needle has adequate length to reach the caudal epidural space in the morbidly obese using a vertical orientation. If the needle is not advanced above the level of the cornua, fluid may not ascend as high in the epidural space. The cephalad extent of flow with this vertical method or with cephalad insertion immediately after penetrating the sacrococcygeal membrane (15) is likely to be volume dependent.
5.4. Conclusion
A positive epidurogram suggestive of successful injection at first needle placement in 90% of participants with 0% intravascular injection suggests that the accuracy and safety of this vertical small-needle caudal epidural injection technique compares favorably with fluoroscopic guidance. The results from this study suggest an alternative approach to conventional techniques. This technique appears easy to learn and may be more comfortable for the patient. It may also be combined with Doppler ultrasound monitoring for an epidural flow pattern.