1. Background
Post-thoracotomy pain syndrome (PTPS) is a pain syndrome that occurs within two months following thoracotomy at the site of the surgical scar. The international association for the study of pain (IASP) has defined PTPS as pain that recurs or persists along a thoracotomy incision for at least two months after surgery (1). The incidence of this syndrome is approximately 50% in thoracotomies (2). PTPS affects patients’ quality of life and also their postoperative condition.
The origin of PTPS is attributed to trauma to and compression of the intercostal nerves, fractured and compressed ribs, inflammation of the chest muscles, or neuromas located on injured nerve endings. In contrast, features of neuropathic pain reported by individuals with PTPS suggest that intercostal nerve injury may be the main contributor (3).
Another issue in chronic pain syndrome is the role of the sympathetic nervous system. A sudden discharge from silent neuromas originating from nerve ending proliferations after an injury could induce neuropathic pain. Sympathetic nerve growth in the dorsal root ganglion due to chronic neuropathic pain may also precipitate sympathetic mediated pain.
Treatment of PTPS is of particular importance not only to keep the patient comfortable but also to minimize postoperative pulmonary complications (4). PTPS often induces psychosocial stress and anxiety or depression. Pre-emptive analgesia is a multi-potential method of preventing pain prior to its occurrence (5). Although some interventions (both procedural and pharmacologic) have been investigated to prevent and treat PTPS, the benefits of these interventions have not yet been determined (6, 7). Dexmedetomidine (dex) and clonidine are α-2 agonists that have analgesic, sedative-hypnotic, and sympatholytic properties (8, 9). In this study, we investigated whether dexmedetomidine could be used to control both hemodynamic changes and PTPS.
2. Objectives
To determine the effect of pre-emptive dex on the incidence of PTPS in patients undergoing coronary artery bypass grafting (CABG).
3. Patients and Methods
3.1. Ethics Declaration
The study was reviewed and approved by the Shahid Beheshti University of Medical Sciences ethics committee and was performed in accordance with its regulations. Information about the study was given comprehensively in both oral and written form to all patients or their accompanying adult. The participants gave their written informed consent prior to their inclusion in the study.
3.2. Patient Selection
This randomized clinical trial enrolled 104 patients who were candidates for elective CABG and randomly assigned them to either the dex group or the control group. Randomization was carried out using numbers that were assigned to each patient by a computer. The patients were diagnosed with PTPS if they described their pain syndrome as a continuous burning and aching in the area of the incision that had persisted for at least two months after thoracotomy based on the IASP definition. All patients were contacted two months after the surgery and interviewed.
Exclusion criteria included bleeding diasthasis either during or after surgery, patients with more than four grafts, patients with hypotension (systolic blood pressure < 90) prior to surgery, cardiopulmonary bypass (CPB) duration of more than two hours, a heart rate less than 50, an intubation time greater than 12 hours, and kidney or liver failure.
In the dex group, 0.5 µg/kg/hour of dexmedetomidine was infused from the initiation of anesthesia until extubation in the intensive care unit (ICU). In the control group, patients received a corresponding volume of 0.9% saline.
3.3. Monitoring and Anesthesia
All customary monitoring procedures were performed, including pulse oximetry, invasive blood pressure monitoring, electrocardiogram, capnography, bispecteral index (BIS), and cerebral oximetry. Anesthesia was administered using the standard method. BIS monitoring was carried out to ensure an adequate depth of anesthesia, and isofluorane maintenance accompanied by fentanyl infusion was continued to maintain a BIS:40 - 60.
Hemodynamic parameters (systolic arterial pressure, heart rate, and peripheral oxygen saturation) were recorded by an anesthesiologist who was blinded to the patient group at 0, 30, 60, and 120 minutes after the infusion of dex had been initiated.
Upon establishing full cardiovascular monitoring, general anesthesia was induced with fentanyl 2 µg/kg, midazolam 0.05 mg/kg, lidocaine, and etomidate 1 - 2 mg/kg until the loss of the eyelid reflex. Orotracheal intubation was facilitated using 0.1 mg/kg Cis-atracurium. Routine airway and ventilator management were used as appropriate for the type of surgery. Anesthesia was maintained with oxygen and isofluorane (1% - 1.2% end-tidal concentration) and a continuous infusion of fentanyl until the patient was transferred to the open-heart ICU. Neuromuscular relaxation was ensured through the continuous infusion of Cis-atracurium 1 - 2 µg/kg/min to maintain 90% - 95% twitch inhibition under inhalational anesthesia.
At the completion of surgery, muscle relaxation was not reversed. Instead, the infusion of dexmedetomidine continued, and the patient was transferred to the ICU. Weaning criteria were if the patients could maintain their oxygen saturation above 97% with a normal breathing rhythm (no tachypnea) and no tachycardia. No patient was extubated until he or she was completely awake and free of arrhythmias and bleeding. All patients were transferred to the post-surgical units in a pain-free state.
The target range for sedation was light or moderate sedation, which is 2 and 3, respectively, on the ramsey sedation scale. The protocol for supplemental sedation if the patient became agitated before extubation was an infusion of midazolam in the control group and an infusion of dex at the same rate of 0.5 µg/kg/hour in the dex group.
3.4. Data Collection
All intraoperative data were collected from patients’ files and monitoring records. Follow-up was performed through a telephone interview. The patients were contacted via telephone two months after surgery. The telephone interview was performed using the Brief Pain Inventory questionnaire, which was based on that used in Brulotte et al.’s study (10). The patients were asked about the presence of pain at their thoracotomy scars, allodynia, and the location and nature of any pain. Pain was evaluated using the numeric rating scale (NRS) during the study. The telephone interview was performed by one of the researchers who was unaware of the patients’ group assignment. Patient satisfaction was assessed using the same questionnaire (brief pain inventory) based on questions with yes/no answers.
3.5. Statistical Analysis
Statistical calculations were conducted using the statistical package for the social sciences (SPSS), version 22 (Chicago, IL, USA). The parametric variables were presented as mean ± SD and were analyzed by t-test or Mann-Whitney U-test; non-parametric variables were assessed with the Chi-Square or Fisher’s exact test. P < 0.05 was considered statistically significant. The sample size was estimated using sample size calculator software with a 95% confidence interval, P = 0.05, a power of 80%, and a frequency of 62% in the experimental group and 37% in the control group for the primary outcome (PTPS) based on the Brulotte study.
4. Results
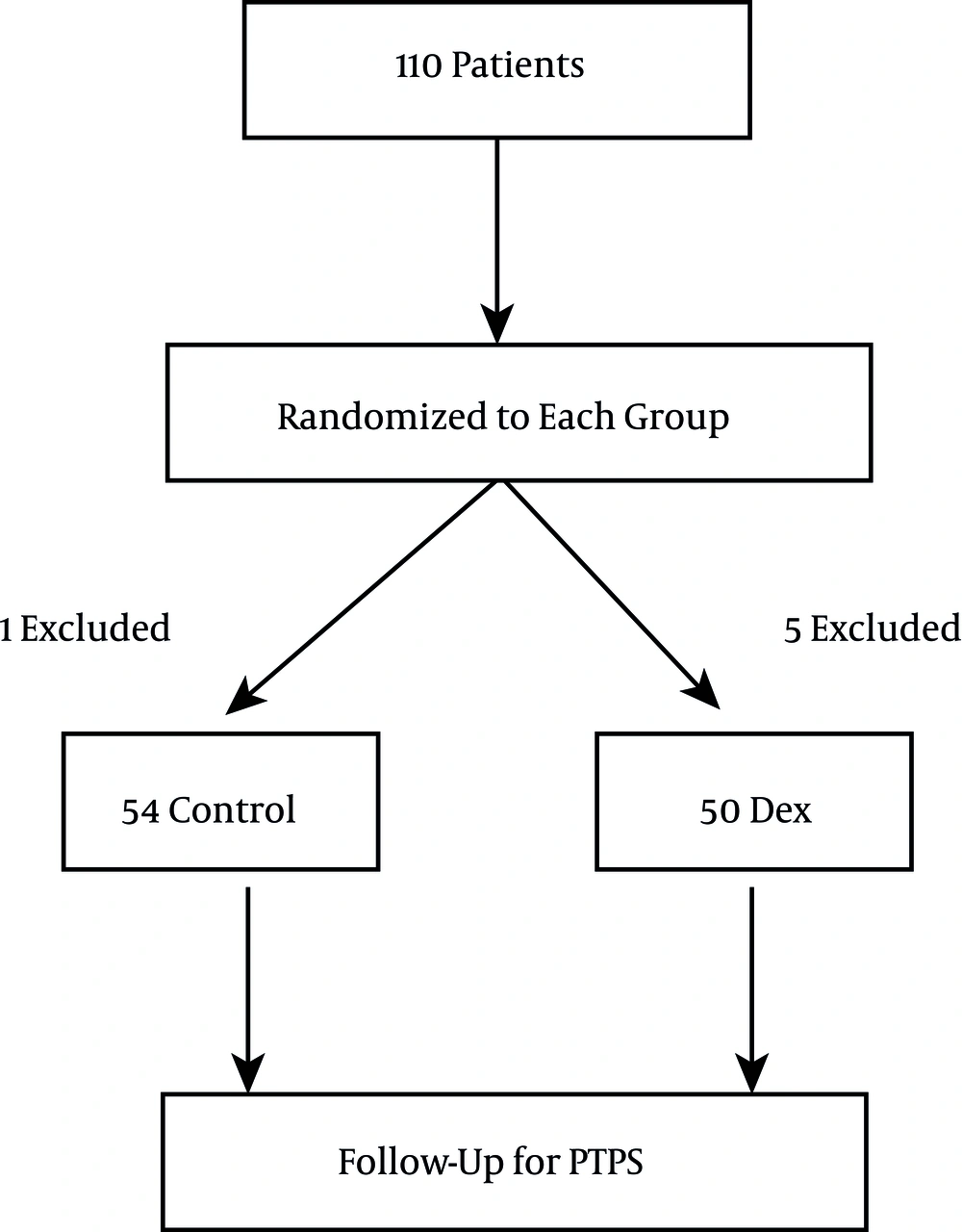
In all, 110 patients were enrolled in this study and randomly assigned to one of the two groups. After exclusions were made, 54 patients remained in the control group, and 50 patients were enrolled in the dex group. A flow diagram of the study is depicted in Figure 1. The age, sex, and body mass index were not significantly different between the two groups of study (P > 0.05) (Table 1).
| Variable | Dexmedetomidine (n = 50) | Control (n = 54) | P Value |
|---|---|---|---|
| Age | 57.3 ± 9.7 | 55.4 ± 8.8 | 0.28 |
| Sex , male/female | 34/16 | 37/17 | 0.39 |
| BMI | 24.6 ± 4.7 | 25.1 ± 4.5 | 0.29 |
| Duration of CPB | 52.6 ± 15.4 | 55.7 ± 16.2 | 0.30 |
| Duration of Surgery | 4.8 ± 2.5 | 4.45 ± 2.7 | 0.24 |
Abbriviations: BMI, body mass index; CPB, cardiopulmonary bypass.
The variables of coronary artery disease (CAD), ejection fraction, and history of chronic disease (including hypertension (HTN), diabetes mellitus (DM), and hypothyroidism) were not significantly different between the two groups included in this study (P > 0.05) (Table 2).
| Variable | Dexmedetomidine (n = 50) | Control (n = 54) | P Value |
|---|---|---|---|
| Number of CADs | 0.057 | ||
| LAD artery | 15 | 12 | |
| 2 vessels | 17 | 14 | |
| 3 vessels | 18 | 28 | |
| Ejection fraction (EF) | 51.7 ± 9.5 | 52.6 ± 8.7 | 0.33 |
| History of chronic diseases | 0.19 | ||
| HTN | 9 | 12 | |
| DM | 12 | 15 | |
| Hypothyroidism | 2 | 1 |
Abbriviations: CAD, coronary artery disease; LAD, left anterior descending; HTN, hypertension; DM, diabetes mellitus.
After two months, the patients were followed up using a telephone interview. The patients’ NRS score if they were experiencing PTPS pain at two months, the presence of allodynia, and the level of patient satisfaction in the two groups of study were compared; the differences were not significantly different (P > 0.05) (Table 3).
| Variable | Dexmedetomidine (n = 50) | Control (n = 54) | P Value |
|---|---|---|---|
| NRS score in PTPS patients, (Mean ± SD) | 5.7 ± 2.1 | 5.5 ± 2.8 | 0.43 |
| Allodynia, No. (%) | 9 (18) | 24 (44) | 0.003 |
| Patient satisfaction, No. (%) | 32 (64) | 37 (68) | 0.35 |
Abbriviation: NRS, numeric rating scale.
The pain score reflects the amount of pain in patients with PTPS and not that of patients who were pain free (corrected in text). Allodynia was significantly higher in the control group compared to the dex group (Table 3). Patient satisfaction was not significantly different between the two groups (P = 0.35).
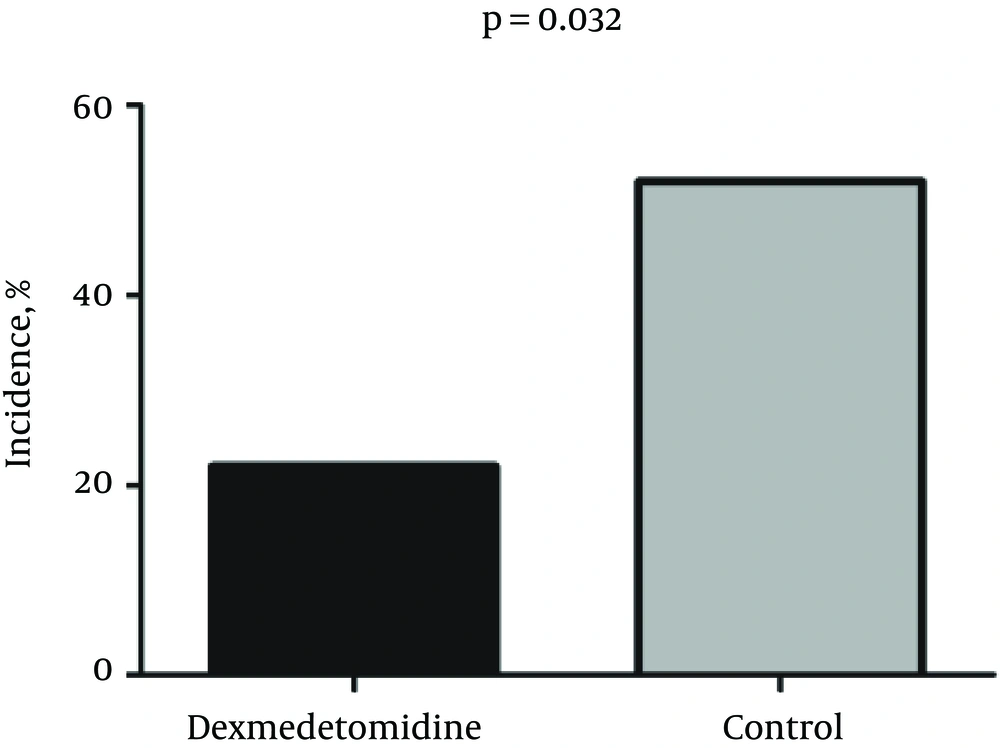
The incidence of PTPS in the dex group was 11/50 (22%) patients, while 28 of 54 (52%) patients in the control group reported PTPS. Chi-square test revealed a significant difference in the incidence of PTPS two months after surgery between the dex and the control groups (P = 0.032) (Figure 2).
5. Discussion
The true incidence and pathogenesis of PTPS is still unknown, and results from human studies vary, which can be reasonably explained by the complexity of the methodology and systematic nature of previous studies that cause several variables to change (11). Our investigation of 104 patients showed that the incidence of PTPS was about 52% in the control group. One previous report was consistent with our study and showed that PTPS occurs in approximately 50% of patients following thoracotomy and is usually mild to moderate; only 5% reported severe symptoms (12). In another study, severe, disabling PTPS was present in 25 (11%) patients: 10/20 (50%) chest wall resections, 5/25 (20%) pleurectomies, and 10/193 (5%) pulmonary resections (13). Out of 149 patients in a different study, the overall incidence of PTPS was 52% (32% mild, 16% moderate, and 3% severe) (14). In an interesting investigation, 70% of patients reported persistent pain a month after surgery, while 41% of patients were still experiencing persistent pain one year postoperatively (15). In another study conducted in 110 patients, the incidence of PTPS was 80% at three months, 75% at six months, and 61% one year after surgery; the incidence of severe pain was 3% - 5% (16).
Due to variations in pathogenesis, several mechanisms have been suggested for PTPS in this patient population. The role of the sympathetic nervous system in the progression of neuropathic syndromes has been noted. Sympathetic overstimulation could induce a sympathetic pain-related syndrome. Increased inflammatory processes in neuropathic pain syndromes might also be aggravated by sympathetic stimulation (17). Sympathetic ganglion blocks have been recommended to treat sympathetic-related neuropathic pain syndromes, such as complex regional pain syndrome (18). Dexmedetomidine exhibits sympatholytic activity and prevents sympathetic stimulation, which could be an effective pre-emptive therapy that hinders the development of neuropathic pain syndrome in PTPS.
Pre-emptive strategies for post-operative pain syndrome are the mainstay of current pain control. However, few studies have investigated pre-emptive therapy. Central nervous system hypersensitization in response to tissue injury may contribute to the development of PTPS (19). Many researchers have focused on methods to prevent central sensitization from occurring through the use of pre-emptive analgesic techniques. Effective pre-emptive analgesia may be more useful than palliative post-surgical therapies (20). Our study of 104 patients indicated that the incidence of PTPS decreased from 52% in the control group to 22% in the dexmedetomidine group.
Some previous reports on pre-emptive analgesia have revealed that ketamine had no effect compared with a placebo in the prevention of PTPS at three and six months postoperatively (21). Another study showed the effects of adding ketamine to fentanyl plus acetaminophen on postoperative pain as a form of patient-controlled analgesia in abdominal surgery (22). Anesthestic drugs have also proven effective on reducing the incidence of PTPS (23). TIVA with propofol and remifentanil may reduce the incidence of PTPS three and six months after surgery compared to inhalational anesthesia (24). In another study that used preoperative morphine, diclofenac, and intercostal nerve blocks, there were no significant differences between the groups at the 12-month follow-up (25).
Treatment of PTPS is still a challenge for clinicians (26). Although it has been proven effective for neuropathic pain syndromes, perioperative pregabalin did not reduce the incidence of PTPS (10). Future research on PTPS should focus on the impact of regional analgesia on central sensitization. Percutaneous intercostal nerve cryoablation may result in a few months of pain relief in cases of intractable PTPS (27); however, pre-emptive analgesia may be more effective (28). Based on the results of our study, the effects of pre-emptive analgesia appeared to be of clinical significance.
Prior to our study, postoperative pain control was not a mainstay of treatment for CABG patients at our hospital. In fact, only suboptimal doses of morphine or non-steroidal anti-inflammatory drugs were prescribed by the surgeon to control postoperative pain. However, our study showed a high incidence of PTPS syndrome in these patients and their corresponding need for pain control, which led to better planning for adequate postoperative pain control.
In conclusion, PTPS is a common problem following CABG, and pre-emptive therapy with dexmedetomidine may reduce neuropathic pain by decreasing the intense input from nociceptive neurons to the central nervous system. The effect of pre-emptive intraoperative dexmedetomidine appeared to be relatively modest in terms of preventing PTPS.