1. Introduction
Symptomatic lumbar spinal stenosis is a prevalent condition affecting approximately 1 in 200 people in the United States over the age of 50 (1). Neurogenic claudication remains a debilitating symptom and once the patient fails a conservative course of therapy, surgical options should be considered. Lumbar epidural steroid injections are frequently used as an interventional modality for patients with symptomatic neurogenic claudication however they rarely achieve a long term benefit (2). Endoscopic and open lumbar laminectomies are the main surgical modalities utilized for patients who do not respond to less invasive procedures. Some of these interventions however require general anesthesia and can be complicated by dural tears with leakage of cerebrospinal fluid (CSF), hematoma and perioperative infections (3). In an effort to decrease these potential complications a minimally invasive lumbar decompression (MILD) procedure was introduced. This procedure treats patients with neurogenic claudication secondary to lumbar spinal stenosis who have ligamentum flavum hypertrophy as a contributing factor. The goal of this procedure is to remove a small part of the laminar bone and partial debulking of the ligamentum flavum. Short term follow-up at 6 weeks appear to be promising with improvement of both pain and function (4). Also there were only minor complications reported. Despite initial euphoria there was evidence that some of the patients required open surgery once the official study period ended (5). Subsequently a new prospective, multi-center, randomized controlled clinical study is presently under way with the goal for a long term; up to two years follow up (6).
Our goal was to develop a safer procedure for removal of the hypertrophic ligamentum flavum with less trauma to the surrounding tissues. As compared to the MILD procedure we did not perform any laminar bone decompression, although that should be an option if bony stenosis is deemed to be present. Our design constitutes an alternative physical method to debulk the ligamentum flavum. Special attachments were designed, allowing us to use a minimally invasive spinal decompression technique with fluid jet SpineJet (HydroCision Inc, Billerica, MA, USA) which is already approved for HydroDiscectomy. This device allows nucleus pulposus removal, whereby tissue is removed due to creation of a high fluid kinetic energy via a Venturi effect in a closed saline circuit.
The procedure was designed for a subgroup of patients with lumbar spinal stenosis in which hypertrophy of the ligamentum flavum is a major contributor. To be eligible, patients must have failed conservative treatments, have predominantly leg pain and have a demonstrable evidence of ligamentum flavum hypertrophy (5 mm or more ) on MRI or other imaging study. The procedure is done under monitored anesthesia care (MAC); the underlying hypertrophic ligamentum flavum is resected with a jet of saline and evacuated by suction. Fluoroscopic guidance was utilized to identify important bony landmarks.
2. Case Presentation
An 85-year-old female was referred to our clinic for low back pain radiating predominantly into the right leg. The pain started gradually one year prior to her initial visit and was affecting her right leg with standing and walking for 30 feet. She failed conservative therapies including physical therapy, home exercises, pain medications and epidural steroid injections.
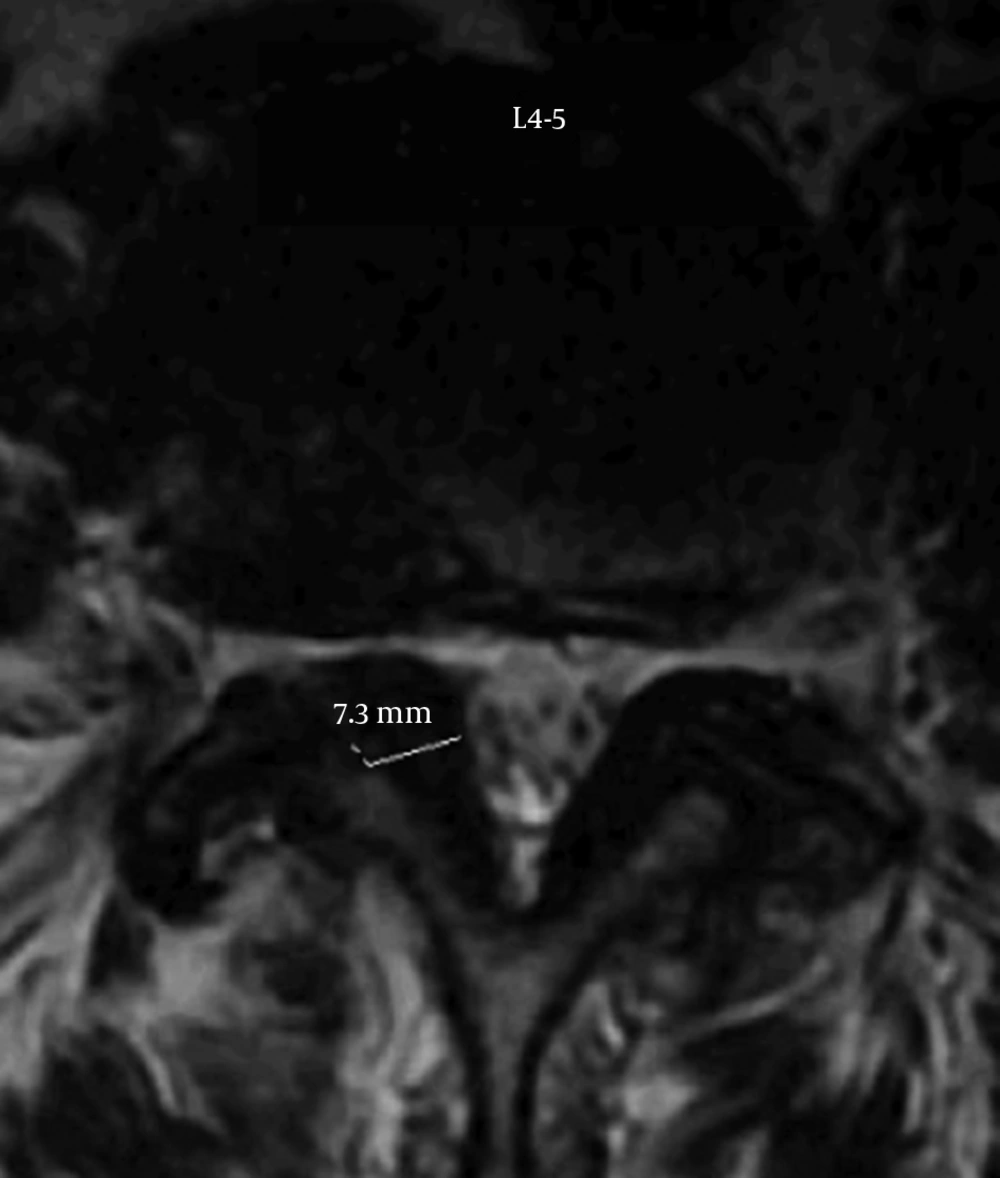
A magnetic resonance imaging scan was performed. The most pertinent findings were at the right L4-L5 level where ligament flavum hypertrophy (7.3 mm diameter), facet hypertrophy and disc bulging produced severe right foraminal narrowing (Figure 1). There was also foraminal narrowing on the left and some central stenosis. However, given that the symptoms were only related to the foraminal narrowing on the right, our strategy was to treat the patient and not the MRI, and to do this with a safe and minimally invasive approach.
The right leg pain numbness and dysesthesia were the most disabling symptoms. Her visual analog score (VAS) was 8/10 for the right leg and 6/10 for the back. The Oswestry Disability Index (ODI) was 44.4 and the Zurich claudication questionnaire (ZCQ) showed a score of 2.35. She was presented with the option of an open decompressive laminectomy, but she opted for the less invasive hydro decompression technique. At that time the MILD procedure was not available due to lack of insurance coverage. She was fully informed that the use of the equipment was off-label and subsequently she was informed of all potential complications related to this new procedure. As this was an off-label use of a device that was already FDA approved for spinal decompression, we did not present it before an institutional review board.
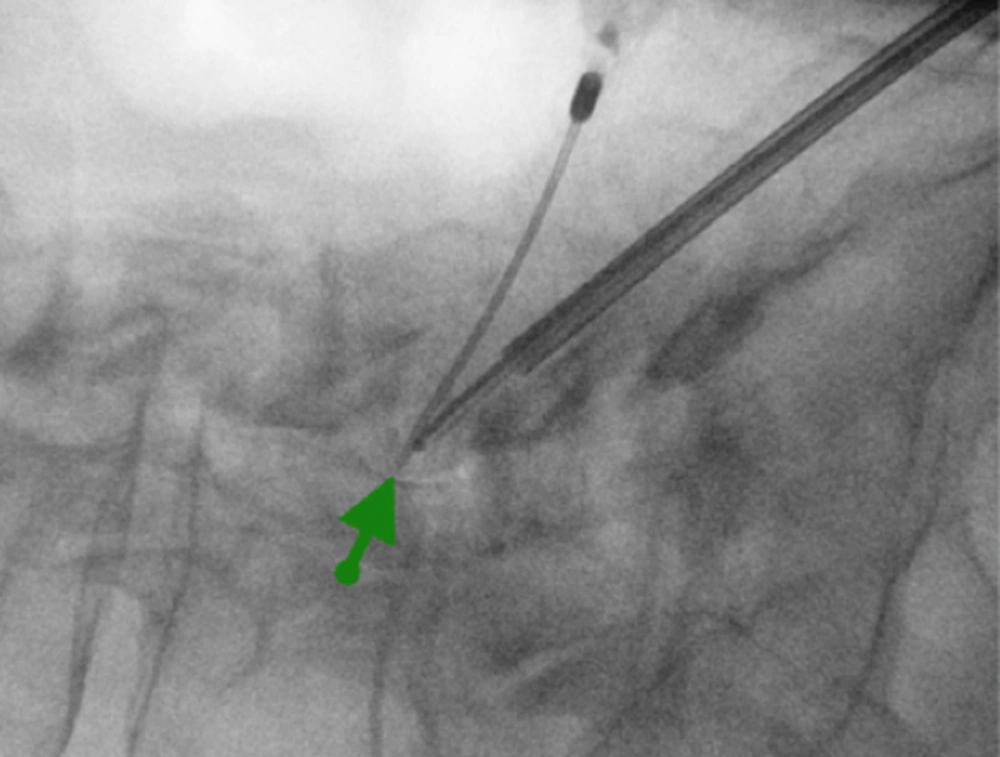
MAC anesthesia was utilized. The patient received 1 GM of Cefazolin preoperatively for prophylaxis. An epidurogram was performed with 3 mL of Iodohexol180 (Omnipaque, GE Healthcare) via an 18 gauge Tuohy needle at the L4-5 level showing evidence of lumbar spinal stenosis. After this a 17 gauge modified epidural needle was inserted posterior to the right S1 foramen and advanced under fluoroscopy lateral to the midline until the tip of the needle rested on the superior surface of the right L5 superior lamina. A guide wire was inserted via the epidural needle which was subsequently removed. After that a cannula and a dilator were placed through a small incision over the guide wire, following which the dilator and the guide wire were removed leaving the cannula in place. Finally the SpineJet resector was inserted into the cannula and advanced to the superior lamina of L5, making contact with the posterior fibers of the ligamentum flavum. For safety reasons an oblique contralateral view was maintained all the time to assure that the resector did not go beyond the posterior aspect of the ligamentum flavum or violate the epidural space. The proper depth of the resector was appreciated based on the epidurogram which allowed us to have a clear demarcation of the epidural space. Small amounts of Iodohexol180 (Omnipaque, GE Healthcare) were injected during the procedure via the adjacent 18 gauge Tuohy needle in order to maintain a good grasp of the epidural borders. It is important to avoid directing the dissecting jet opening in a perpendicular plane to the epidural space as this can produce dural tears. Hydro resection was performed for a total of 90 seconds. At the end of the procedure an epidurogram was again performed showing improved flow at the L4-5 epidural space (Figure 2).
3. Discussion
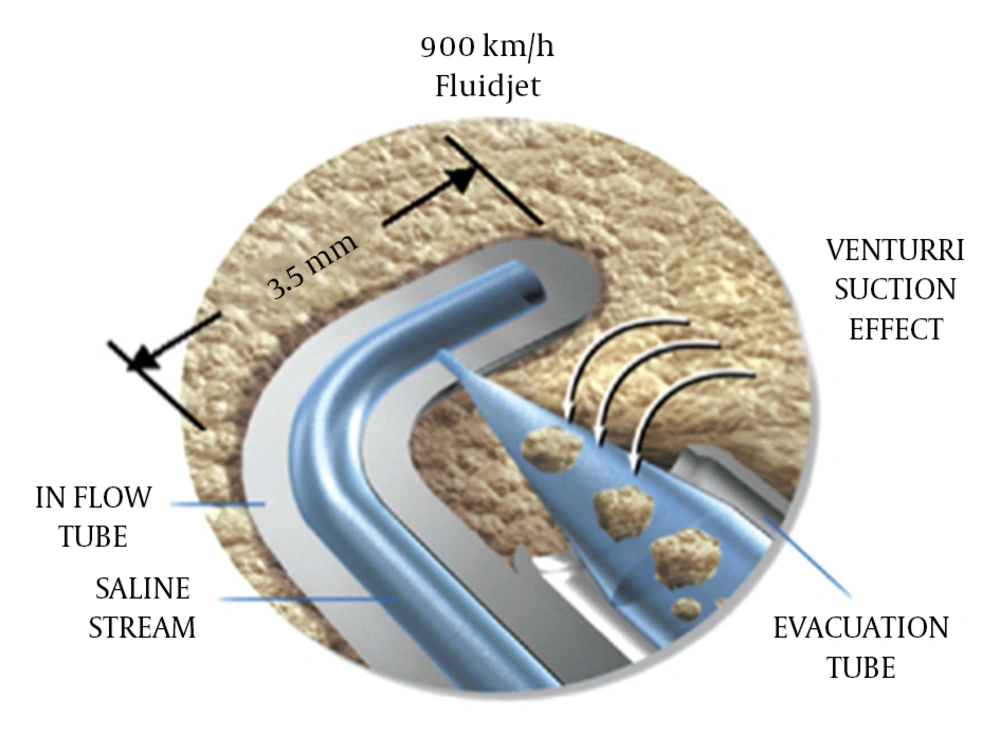
The SpineJet Hydrosurgery system was initially developed for decompression of herniated but contained lumbar discs (7). The system jets saline fluid with high velocity (900 km/h) to cut, ablate and evacuate nucleus pulposus. The resulting debris is removed through an adjacent tube, using a Venturi suction effect (Figure 3).
We hypothesized that in patients with symptomatic lumbar spinal stenosis the SpineJet system could be used to resect and remove ligamentum flavum. If the primary source of the stenosis is the overgrowth of ligamentum flavum, the need for removal of laminar bone may not be essential; however this remains an open question. Our laboratory experience with cadaver dissection showed that 90 seconds was sufficient time to safely remove an adequate amount of ligamentum flavum. It is yet unclear if the total amount of time allowed for the removal of tissues is equally important to how many times the resector is rotated between the caudad and cephalad lamina.
The VAS, ODI and ZCQ were calculated at 3 months, 6 months and one year after the procedure (Table 1). The patient was evaluated in the office by a physician for each specific visit. VAS dropped from preoperative values of 8 in right leg, 6 in back, to 0 and 1 respectively at one year. ZCQ decreased from 2.35 to 1.47, and ODI improved from 44.4 to 2.2. The severity of her symptoms, physical function and social engagement were significantly improved one year after the procedure. Because there were no immediate or long term complications in this off label application of the SpineJet system a MRI study of the lumbar spine was not obtained at the end of the follow-up period. This criticism was actually brought on regarding the published MILD studies.
| Value | VAS for the Leg | VAS for the Low Back | ZCQ | ODI |
|---|---|---|---|---|
| Before the procedure | 8 | 6 | 2.35 | 44.4 |
| 3 months follow up | 1.5 | 1.5 | 1.29 | 2.2 |
| 6 months follow up | 2.5 | 4.5 | 1.64 | 2.2 |
| One year follow up | 0 | 1 | 1.47 | 2.2 |
Percutaneous lumbar hydro decompression with the SpineJet system has a potential use for some patients with lumbar spinal stenosis. Like any other minimally invasive surgery it has the advantages of less tissue trauma and potentially decreased morbidity, providing that it can deliver the originally set up clinical outcomes. Clearly the modality requires further validation in clinical studies with a large number of patients in order to fully assess its safety and efficacy.