1. Background
Postoperative pain management is one of the most important roles of an anesthesiologist. Poorly controlled postoperative pain usually leads to peripheral and central sensitization, which may evolve into chronic pain syndromes. Achieving effective and adequate postoperative analgesia is successfully providing an acceptable level of sedation and early ambulation with low complications (1).
Multimodal analgesia is a new approach that uses different drugs and techniques to reduce the total dose of any one drug, which reduces the risk of side effects (2). Opioids are the cornerstone of postoperative pain control, but the combination of nonopioid analgesics, such as acetaminophen, nonsteroidal anti-inflammatory drugs, and ketamine, with opioids provides an analgesic-sparing effect and decreases side effects (3, 4). Ketamine works by blocking the N-Methyl-D-Aspartate (NMDA) receptors has an antinociceptive effect that has been used in perioperative analgesia (5, 6).
In several meta-analyses, the preoperative administration of small doses of ketamine (20 - 60 mg) decreased opiate consumption and side effects, such as postoperative nausea and vomiting, after surgery. It also inhibited nociceptive central sensitization and attenuated acute tolerance after opiate administration (7).
Tramadol is a synthetic analogue that binds to μ-opiate receptors and inhibits monoaminergic neurotransmitters (norepinephrine and serotonin) reuptake. Tramadol is a weak opioid, and its local anesthetic effects have been demonstrated in several studies (8, 9). It was used for the management and prevention of intra-articular pain after arthroscopic knee surgery (10).
2. Objectives
The postoperative analgesic effects of the subcutaneous wound infiltration with a combination of ketamine and tramadol have not been studied previously. Therefore, the aim of this study was to assess the analgesic effects of small doses of this combination following a pyelolithotomy after renal surgery.
3. Methods
Following the approval of the institutional ethics committee, a double-blind randomized clinical trial was carried out in the urology unit of Sina hospital, which is affiliated with Tehran University of Medical Sciences. This study was registered as IRCT201501243773N12 in the Iranian Registry of Clinical Trials. The convenience sampling method was used.
3.1. Sample Size
Estimates of the number of patients that were prescribed a specific dose of medication (meperidine) in the 24 hours after surgery:
- Patients without intervention: 10
- Tramadol: 5
- Ketamine: 3
- Ketamine + tramadol: 1
Tramadol prevented the prescription of five doses of meperidine and ketamine prevented the prescription of seven doses of meperidine (compared to those without intervention). Therefore, ketamine and tramadol can be expected to prevent the prescription of six doses of meperidine: (5 + 7) / 2. Our hypothesis is that the combination of ketamine and tramadol may interact and that the coadministration of both will prevent nine doses of meperidine (10 - 1). This means that we will have three doses higher than what is expected if the two drugs act independently. We used 9 and 6 for means and 2 for SDs. The sample size used in this study was 16 patients in each of the four groups with α = 0.05 and β = 0.2.
Informed consent was obtained from the patients. All 18 - 80-year-old patients classified as American Society of Anesthesiologists physical status I - II who underwent elective pyelolithotomy surgery during 2013 - 2014 were enrolled in the study. Those excluded from the study were patients with liver disease; renal function impairment (creatinine > 2 mg/mL); a history of opioid addiction; an allergy to tramadol; a history of seizure disorder; any contraindications to ketamine or tramadol, such as hypertension, ischemic heart diseases, psychologic disorders, or seizure; and those who were not willing to participate in the study.
A pyelolithotomy is an operation in which renal surgery is performed via a large subcostal incision in the flank. In the operating room, 0.04 mg/kg of midazolam and 2 μg/kg of fentanyl were used as the premedication for all patients. Anesthesia induction was achieved using 4 - 5 mg/kg of thiopental sodium, 0.5 mg/kg of atracurium, and 1.5 mg/kg of lidocaine. Isoflurane with a minimum alveolar concentration of 1 and 100% O2 were maintained during the anesthesia period. After acceptable anesthesia was achieved, a Foley catheter was inserted. Patients were placed in a flank position during surgery. Bispectral index was maintained between 40 and 50. Until 30 minutes before the termination of the operation, 0.7 μg/kg of fentanyl was injected in patients who experienced a 20% increase in blood pressure or heart rate (compared with baseline values measured in the ward). Patients who required higher doses of opioids were excluded from the study. At the end of the surgery, isoflurane was discontinued.
The patients were randomly divided into four equal groups using sealed envelopes, which were prepared by an anesthetic nurse unaware of the objectives of the study. The same nurse also prepared and labeled similar syringes containing either normal saline or one the study medications:
- 10 mL of saline solution (saline group).
- 1 mg/kg of ketamine in 10 mL of saline solution (K group).
- 1 mg/kg of tramadol in 10 mL of saline solution (T group).
- 0.5 mg/kg of ketamine plus 0.5 mg/kg of tramadol in 10 mL of saline solution (K/T group).
At the end of the operation, one of these medications was injected subcutaneously at each patient’s wound site by a surgeon. After extubation, patients were transferred to the postanesthesia care unit (PACU) and were carefully observed until discharge.
In order to achieve the primary objective of this study, each patient’s pain scores were measured at the time of their arrival in the PACU; 5, 10, 15, and 30 minutes after their arrival; and 1, 6, 12, and 24 hours after their operation using a 10-cm VAS score.
In order to achieve the secondary objectives of this study, each patient’s sedation score was assessed during the first 30 minutes after arriving to the PACU using the Ramsay sedation scale (which uses scores with a range of 0 - 6) simultaneously with their pain scores. Additionally, heart rate and mean arterial pressure were recorded before the surgery, at five-minute intervals throughout the surgery, and during each patient’s stay at the PACU. Any complications the patients experienced, such as nausea, vomiting, and hallucinations, were recorded during recovery.
Rescue analgesia was given intravenously (titratable bolus doses of meperidine up to 0.5 mg/kg) during the first 24 hours after the surgery upon each patient’s demand for more pain control.
The duration of surgery was defined as the interval between the first surgical incision and the last surgical suture.
3.2. Statistical Analysis
The statistical analysis of data was done using IBM SPSS 20. Data were presented as mean ± SD or number (%) where appropriate. Quantitative data were compared between the four groups with the aid of a one-way ANOVA. A chi-squared analysis was used to compare the incidence of variables when appropriate. A Kolmogorov-Smirnov test was used to test the normality of the quantitative data. Repeatedly measured quantitative data were compared between and within groups using a repeated measures ANOVA. The violation of equality of covariance matrices and violation of the assumption of sphericity were verified. Due to the violation of Mauchly’s test for sphericity, the Greenhouse-Geisser correction was used. The Bonferroni correction was used for homogeneity and for adaptation for comparison. A P value < 0.05 was considered statistically significant.
4. Results
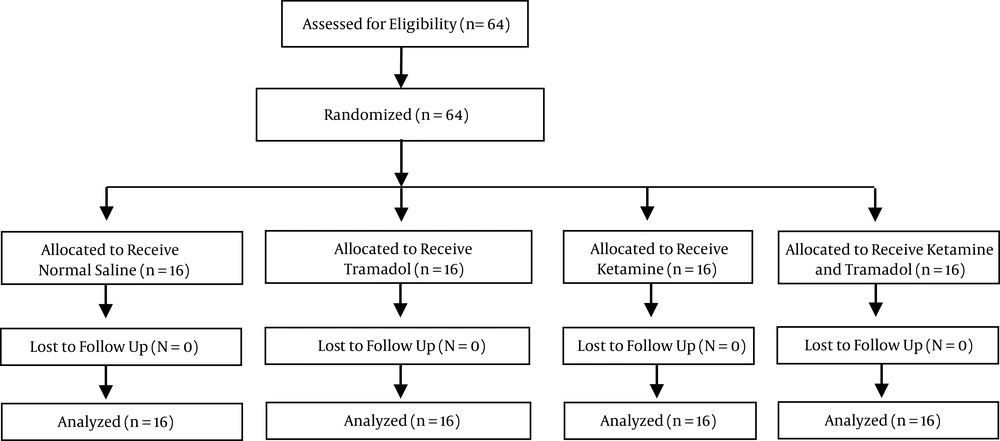
The enrollment flow chart of the patients in this study is displayed in Figure 1. Sixty-four patients, 36 of whom were male (56.3%) and 28 of whom were female (43.8%), with a mean age of 42.86 ± 14.43 years and an age range of 18–80 years were studied over 16 months. There were no significant differences between the demographic variables, surgery duration, and opioid consumption of each group (Table 1).
| Variables | Normal Saline | Ketamine | Tramadol | Ketamine + Tramadol | P Value |
|---|---|---|---|---|---|
| Age, y (mean ± SD) | 40.56 ± 14.42 | 42.38 ± 15.954 | 49.00 ± 16.067 | 39.50± 9.791 | 0.245 |
| Sex | 0.11 | ||||
| Male | 7 | 11 | 9 | 10 | |
| Female | 9 | 5 | 7 | 6 | |
| Surgery duration, minute (mean ± SD) | 109.38 ± 29.99 | 117.94 ± 30.73 | 123.75 ± 38.05 | 126.88 ± 43.162 | 0.536 |
| Fentanyl dose, µg | 201 ± 14.5 | 189 ± 19.8 | 219 ± 17.6 | 210 ± 15.8 | 0.55 |
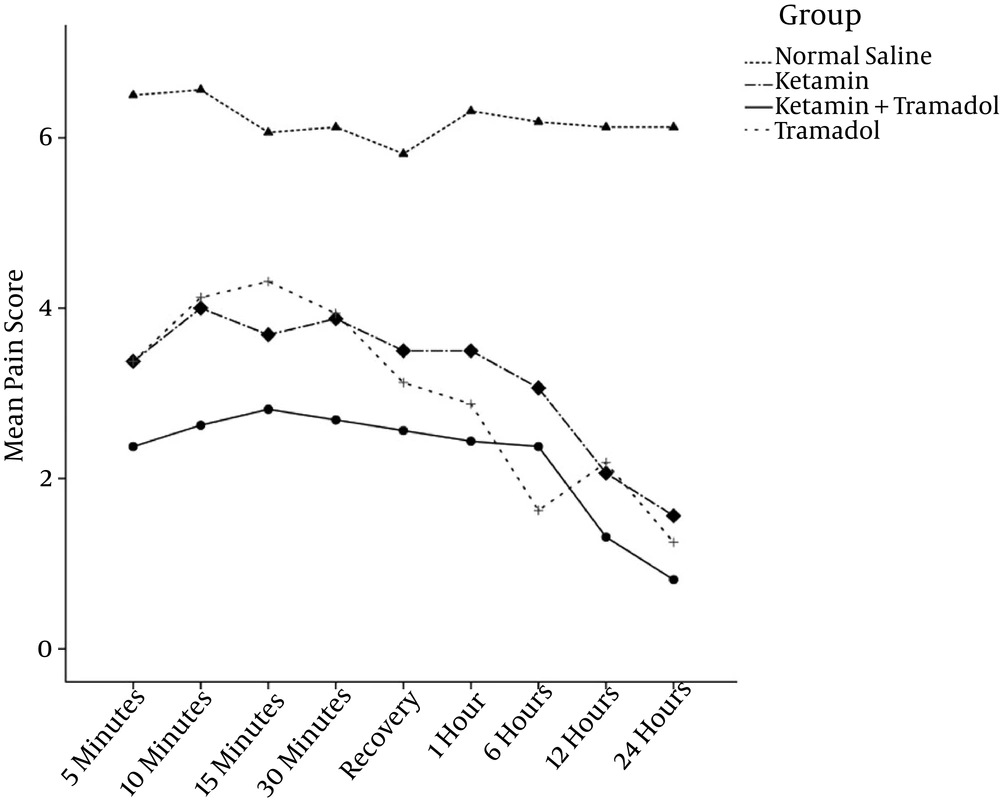
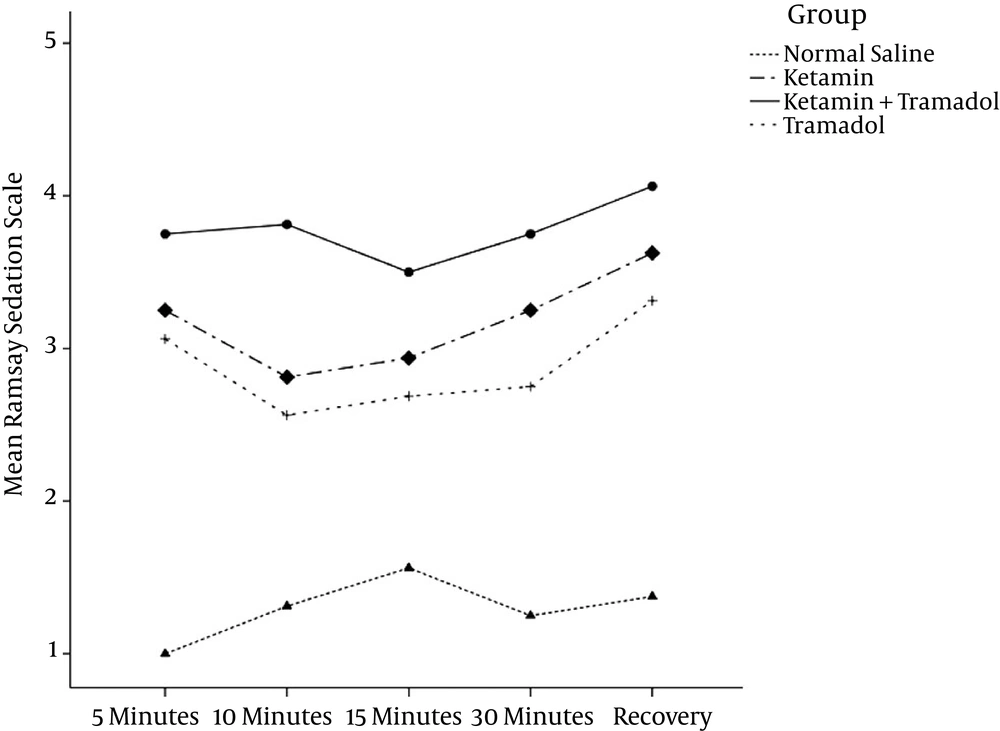
There were significant differences in the pain assessment and sedation scores for different groups and assessment times (P < 0.001), as shown in Table 2, Figures 2 and 3.
The patients’ meperidine requirement during the first 24 hours was lower in the K, T, and K/T groups when compared with the saline group (Table 2).
Patients in the K/T group had significantly lower pain scores compared to those in the K and T groups. There were no side effects noted in any group.
| Variables | Normal Saline | Ketamine | Tramadol | Ketamine + Tramadol | P Value |
|---|---|---|---|---|---|
| Pain score in recovery | 7.2 ± 1.3 | 3.2 ± 1.2 | 3.5 ± 1.4 | 2.2 ± 1.3c | 0.001 |
| Pain score in ward | 6.3 ± 1.5 | 3.3 ± 1.1 | 3.4 ± 1.2 | 1.9 ± 1.1c | 0.001 |
| Sedation score in recovery | 1.5 ± 0.9 | 3.4 ± 1.2 | 3.2 ± 1.2 | 3.9 ± 1.3c | 0.001 |
| Postoperative meperidine requirement, mg | 46.4 ± 6.8 | 25.6 ± 2.6 | 27.5 ± 4.2 | 29.4 ± 3.2c | 0.001 |
| Heart rate in recovery | 93 ± 3.6 | 81 ± 2.5 | 76 ± 2.1 | 78 ± 3.2c | 0.001 |
| MAP in recovery, mmHg | 103 ± 11.3 | 98 ± 10.2 | 106 ± 11.3 | 95 ± 12.5 | 0.19 |
Abbreviation: MAP: mean arterial blood pressure.
aValues are expressed as mean ± SD.
bThe ketamine group received 1 mg/kg of ketamine, the tramadol group received 1 mg/kg of tramadol, the ketamine + tramadol group received 0.5mg/kg of ketamine plus 0.5mg/kg of tramadol s.c., and the saline group received a similar volume of normal saline s.c.
cP < 0.05 vs. the ketamine + tramadol group and the saline group.
5. Discussion
The main finding of this study was that the subcutaneous wound infiltration of a combination of ketamine and tramadol reduced postoperative pain scores significantly more than a normal saline solution, a ketamine solution, or a tramadol solution in patients undergoing a pyelolithotomy. Combined tramadol and ketamine infiltration was also effective at reducing meperidine consumption in the first 24 hours during the postoperative period. The level of sedation was, however, increased in the K, T, and K/T groups when compared to the saline group.
The lower pain scores in the K/T group should be interpreted together with the significant meperidine-sparing effect. Reduced meperidine consumption may relate to the direct analgesic actions of ketamine mediated via the mu receptor and the inhibitory action of the excitatory glutamatergic NMDAR receptors (7).
The combination of opioids with other drugs, like ketamine, in PCA is currently a cornerstone of postoperative analgesia. In a review article, the addition of ketamine to opioids for intravenous PCA in thoracic surgery was better than opioids alone, but this combination was not significant for orthopedic or abdominal surgeries (11). In a study by Imani et al., the addition of ketamine to intravenous fentanyl and acetaminophen PCA was investigated, which revealed no significant analgesic effects for abdominal surgical procedures (12).
The subcutaneous administration of analgesic drugs is a technique for postoperative pain management. These drugs are usually administered at the incision site to control pain.
Many studies have shown the peripheral local anesthetic effects of tramadol and ketamine (13-15). According to these studies, the suggested sites of action for this drug are the nerve endings and a possible associated central effect. Safavi et al. showed that either the subcutaneous infiltration of 2 mg/kg of ketamine or the intravenous injection of 1 mg/kg of ketamine approximately 15 minutes before surgical incision could provide an adjunctive analgesia during the first 24 hours after surgery in patients undergoing a cholecystectomy (16). Another study showed that the subcutaneous infiltration of ketamine at an incision site after a Caesarean section produced less pain than a placebo (17). Nejati and colleagues reported that intranasal ketamine is an effective agent for reducing the pain of nasogastric tube insertion (18). They reported less pain intensity and less analgesic consumption than patients who were given a placebo. In addition, topically applied ketamine in conjunction with lidocaine gel resulted in a tolerable condition for a rigid cystoscopy (19). Khajavi et al. reported that subcutaneous wound infiltration with tramadol (2 mg/kg) following a pyelolithotomy is associated with a lower incidence of nausea and vomiting, a reduced need for opioids, a decrease in pain scores, and a higher sedation level in recovery (20). In our study, we administrated small doses of tramadol (0.5 – 1 mg/kg), which had the same analgesic effect as Khajavi et al.’s study (20).
In previous trials, preincision tramadol and ketamine injections were separately examined, but in this clinical trial, we used a low dosage combination of both (21, 22).
Many published trials have been reviewed the intravenous addition of ketamine to opioids, such as morphine and tramadol, for acute postoperative pain management (23, 24). All these trials showed that a small dose of ketamine was a useful addition to tramadol and morphine after major surgery (25). The major complication of ketamine was hallucinations, which had a reported incidence of 23% - 33% after morphine and ketamine infusion (26, 27). In our study, due to the single injection of ketamine and tramadol, no patients experienced hallucinations or any others psychologic complications.
Ketamine has an elimination half-life of 100 - 180 minutes. However, prolonged analgesic action after a single injection may be due to ketamine’s active metabolite norketamine, its anti-inflammatory effects, its inhibition of nociceptive central hypersensitization, and its attenuation of acute tolerance to opiate administration (28).
The current study is subject to one limitation: We did not measure the plasma levels of ketamine and tramadol to evaluate the link between the blood concentration of these drugs and the resulting analgesic effects.
In conclusion, the subcutaneous infiltration of a small dose of a combination of ketamine and tramadol at an incision site could control pain intensity with a low opioid requirement during the first 24 hours after renal surgery.