1. Background
Subarachnoid block is the preferred technique for most lower segment caesarean sections in patients with pregnancy-induced hypertension (PIH). However, this procedure has only a limited duration of analgesia and causes maternal hypotension perioperatively, which may be deleterious in PIH patients. The discovery of various spinal receptors like α2-adrenergic, cholinergic, opioid, N-methyl-D-aspartate, gamma-aminobutyric acid (GABA), and benzodiazepine receptors triggered the usage of drugs like clonidine, neostigmine, opioids, ketamine, and midazolam for their synergistic effect with hyperbaric bupivacaine (0.5%) in prolonging the duration of analgesia (1, 2). In addition, the efficacy of intrathecal amitriptyline and doxepin, which belong to different classes of drugs, have been studied in rats (3).
Local anesthetics with opioids demonstrate significant synergy (4). Spinal opioids are effective but do produce adverse effects, like respiratory depression, urinary retention, nausea and pruritus, which may not be desirable in PIH patients. Furthermore, the single administration of an opioid may induce a long-lasting increase of threshold pain sensitivity, leading to delayed hyperalgesia (5).
The benzodiazepines are used primarily for anxiolysis, amnesia, and sedation (6). The discovery of benzodiazepine receptors in the spinal cord triggered the use of intrathecal midazolam for analgesia (7). Several investigations have shown that the intrathecal or epidural administration of midazolam produces a dose-dependent modulation of spinal nociceptive processing in animals and humans and is not associated with neurotoxicity, respiratory depression, or sedation (8). Another study demonstrated the analgesic benefit of midazolam in the early postoperative period following caesarean section. As a result, various studies have shown that the analgesic effect of intrathecal bupivacaine is enhanced by intrathecal midazolam without producing significant side effects (9, 10). Various researchers have evaluated the effectiveness of intrathecal midazolam in postoperative analgesia in normal caesarean patients (11, 12). However, the effect of intrathecal midazolam has not been evaluated in PIH patients. Therefore, this study was designed to determine the analgesic efficacy and also the characteristics of the spinal block achieved using 2 mg midazolam along with 2 mL of 0.5% hyperbaric bupivacaine in PIH patients scheduled for elective caesarean section.
2. Objectives
The primary objective of this randomized, double-blind, and placebo-controlled clinical trial was to examine the duration of postoperative analgesia until the first requirement for analgesic supplementation (rescue analgesia). The secondary objectives included the assessment of the onset time of sensory and motor blocks, the duration of the blocks, hemodynamic variables, the incidence of hypotension, vasopressor requirements, bradycardia, and adverse events, such as sedation and postoperative nausea and vomiting.
3. Methods
This study was undertaken in the government general hospital attached to the ESIC Medical College in Gulbarga. After approval of the Institutional Ethical Committee and written informed consent were obtained, 60 patients who ranged in age from 18 - 40 years old with an American Society of Anesthesiologists physical status of I or II and who had been diagnosed with PIH, placed on regular treatment, and scheduled for elective caesarean section under spinal anesthesia were enrolled in this prospective, double-blind, randomized control study. Exclusion criteria included patients of a height less than 150 cm, any significant cardiovascular or hepatorenal diseases, an altered coagulation profile, mental disorders, a history of convulsions, and any contraindications for a regional block, like local infection, HELPP syndrome, or a known drug allergy to local anesthetics.
The patients were randomly allocated using a computer-generated randomization list to one of two groups that contained 30 members each. Blinding was achieved through the use of equal amounts of drugs (2.4 mL), while each syringe was labeled BC (control group) and BM (midazolam group) according to its contents. Identical coded syringes were prepared by personnel who were not involved in the study and then were randomly handed to the anesthetists, who were unaware of the identity of the drugs. The BC group received 10 mg bupivacaine combined with 0.4 mL distilled water, and the BM group received 10 mg bupivacaine combined with 2 mg of preservative-free midazolam (Mezolam, Neon labororatories, Andheri East, Mumbai).
A thorough pre-anesthetic evaluation was carried out the day before surgery, and the required clinical and laboratory investigations were done accordingly. The procedure was explained to each patient, and premedication was provided, which consisted of a 0.5-mg alprazolam tablet and a 150-mg ranitidine tablet to be taken orally at bedtime the night before surgery. All patients were kept nil per os for eight hours prior to surgery. A peripheral intravenous (IV) line was secured with an 18-gauge cannula in one of the upper limbs. All patients were preloaded with 10 mL/kg of Ringer lactate solution 30 minutes prior to the subarachnoid block. All patients received an IV injection of 50 mg ranitidine and 4 mg ondansetron, and monitors were connected to record their heart rate (HR), non-invasive systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), continuous electrocardiogram (ECG) and oxygen saturation (SPO2). Later, a 25-gauge Quincke’s needle was inserted intrathecally using an aseptic technique via a midline approach into the L3-L4 interspaces by the anesthetist, who was unaware of patient assignment, while the patient was in the left lateral position. After a successful dural puncture, the anesthetic solution was injected. The patient was immediately placed in the supine position, and a wedge was used to support the right hip and remained in place until the maximum level of sensory block was achieved. At this point, if a change in position were required, it was allowed. All patients received oxygen supplementation via Hudson’s mask at a rate of 4 L/ minutes.
Postoperatively, the patients were observed for the duration of analgesia using the verbal rating pain (VRS) scale from 0 - 10 (with 0 being no pain and 10 being the most severe pain imaginable) at 1-h intervals until supplementary analgesia was required. Rescue analgesics were given in the form of a tramadol injection (100 mg IV) as well as an injection of Diclofenac (1.5 mg/kg/mg intramuscularly (IM)) once the VRS was recorded as 4 or more.
Assessments of the sensory and motor blocks were taken at the end of each minute until the maximum level of block (T4) was achieved and were assessed using a short, beveled 22-gauge needle and tested at the midclavicular line on the chest, trunk, and legs on either side. The duration of the sensory block was defined as the time for regression of the sensory block from the maximum block height to the L-1 dermatome as evaluated by a pinprick. The onset of the sensory block was taken from the time of induction of spinal anesthesia until the time required for the level of the sensory block to reach the T10 dermatome. The motor block was assessed using the modified Bromage score (0: no motor loss; 1: inability to flex the hip; 2: inability to flex the knee; and 3: inability to flex the ankle); the onset of the motor block was defined as the time that elapsed from the intrathecal injection to a Bromage block 1, whereas the duration of the motor block was assumed when the modified Bromage score was zero. HR, SBP, DBP, MAP, and SPO2 measurements were obtained at 2, 5, and 10 minutes after spinal anesthesia as T2, T5, and T10, respectively, and also every 5 minutes thereafter until the end of surgery.
In our study, hypotension was defined as a more than 20% decrease in the SBP from the baseline. It was treated with IV fluids and a 3-mg mephentermine injection at incremental doses. Bradycardia was defined as a decrease in the pulse rate to less than 60 beats per minutes and was treated with an IV injection of 0.6 mg atropine sulfate. The sedation scores were recorded using the observer’s assessment of the alertness/sedation scale (OASS) as shown in Table 1.
| Responsiveness | Speech | Facial Expression | Eyes | Composite Score |
|---|---|---|---|---|
| Responds readily to name spoken in a normal tone | Normal | Normal | Clear, no ptosis | 5 (alert) |
| Lethargic response to name spoken in a normal tone | Mild slowing or thickening | Mild relaxation | Glazed or mild ptosis (less than half the eye) | 4 |
| Responds only after name is called loudly or repeatedly | Slurring or prominent slowing | Marked relaxation (slacked jaw) | Glazed or marked ptosis (half the eye or more) | 3 |
| Responds only after mild prodding or shaking | Few recognizable words | 2 | ||
| Does not respond to mild prodding or shaking | 1 (sleep) |
The sample size was calculated on the basis of previous studies for detecting a clinically significant difference of 30% in the duration of analgesia and assumed a power of 80% and a significance level of 5%. Statistical comparisons were carried out using Chi-square or Fisher’s exact test and independent Student’s t-test where appropriate. A P value of < 0.05 was considered statistically significant.
4. Results
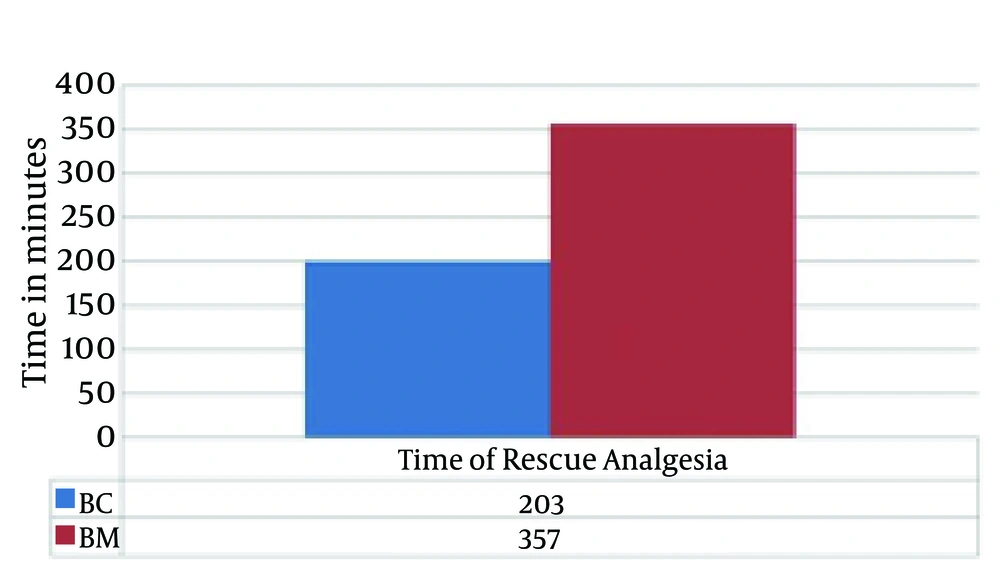
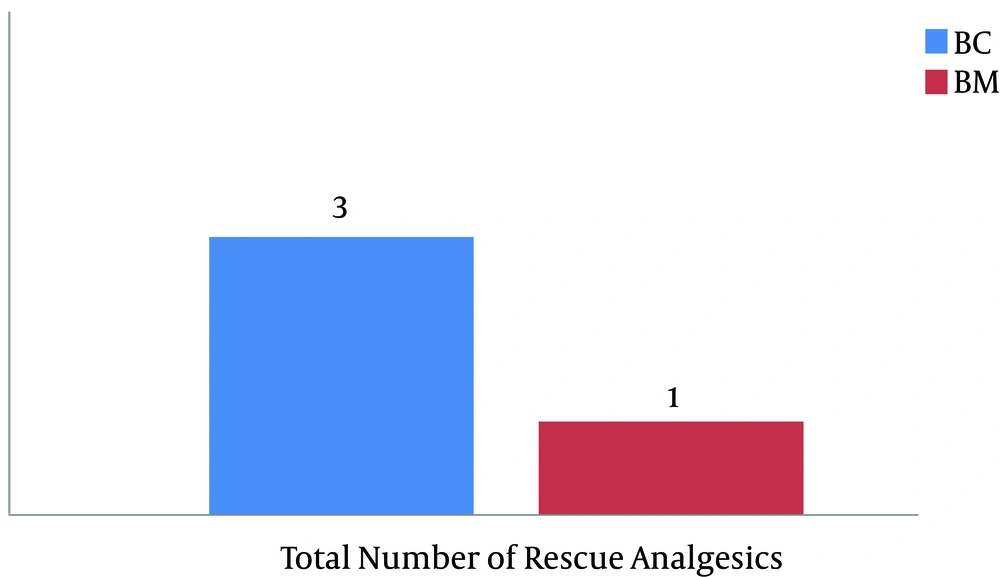
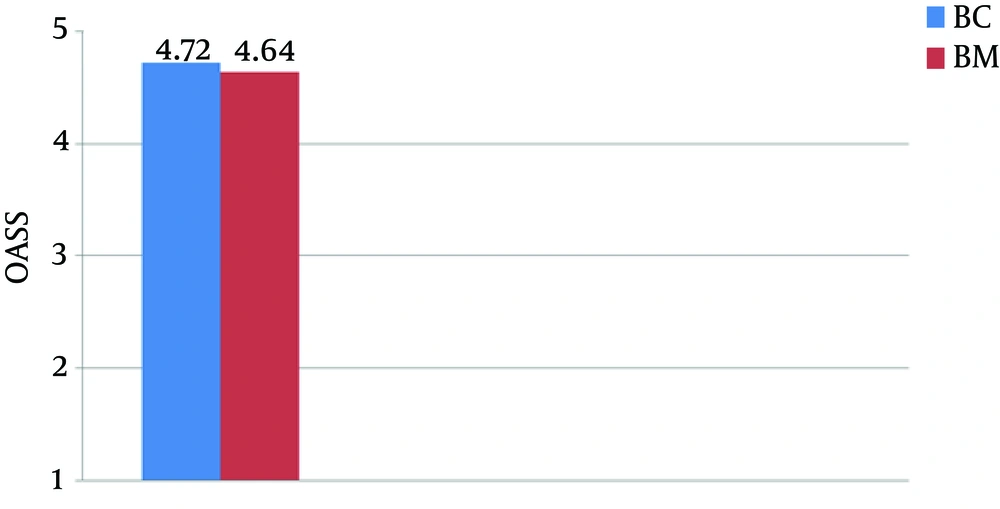
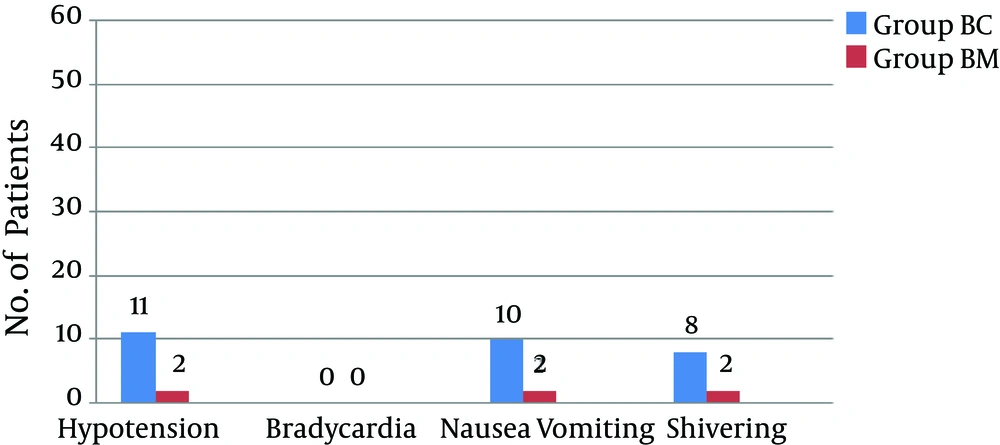
There were no significant differences in the demographic profiles between the two groups (Table 2). The duration of postoperative analgesia was significantly prolonged in the midazolam group compared to the control group (BC: 201.5 ± 1.83 vs. BM: 357.6 ± 9.74, P < 0.01); the number of times rescue analgesics were administered in the midazolam group was significantly less (Figures 1 and 2). The characteristics of the sensory and motor blocks are summed up in Table 3. The mean onset times for sensory and motor blocks were significantly faster in the midazolam group compared to the control group (P < 0.01). Similarly, the time required to attain maximum sensory and motor blocks was significantly faster in the midazolam group compared with the control group (P < 0.05). The duration of analgesia was also significantly prolonged in the midazolam group compared to the control group (260.6 ± 22.45 minutes. vs. 170.8 ± 21.17 minutes). However, the duration of the motor block was comparable in both groups, as was the sedation score (Figure 3). The incidence of hypotension was significantly higher in the control group (36.6%) when compared to the midazolam group (6.66%) and required a repeated dose of mephentermine. There was also a significant reduction in the incidence of side effects (Figure 4).
| Group BC | Group BM | P Value | |
|---|---|---|---|
| Age, y | 24.34 ± 3.77 | 26.06 ± 4.44 | ns |
| Weight, kg | 59.72 ± 4.81 | 59.58 ± 5.36 | ns |
| Height, cm | 159.88 ± 5.14cm | 160.04 ± 5.53 cm | ns |
| Duration of surgery, min | 48.44 ± 14.63 | 51.46 ± 13.93 | ns |
| ASA PS I/II | 12/18 | 14/16 | ns |
Abbreviations: ASAPS, American society of anesthesiologists physical status; cm, centimeter; Kg, kilogram; min, minutes; ns, not significant.
| Parameters | Group BC | Group BM | P Value |
|---|---|---|---|
| Onset time of sensory block, min | 2.96 ± 0.5 | 1.10 ± 0.35 | < 0.01 |
| Time to attain maximum sensory level (T4) | 7.6 ± 1.49 | 4.1 ± 0.85 | < 0.01 |
| Onset time of the motor block (Bromage 1) | 4.04 ± 0.69 | 1.23 ± 0.46 | < 0.01 |
| Time required to attain the maximum motor block (Bromage 3) | 6.42 ± 0.90 | 3.04 ± 0.85 | < 0.05 |
| Duration of the sensory block | 170.8 ± 21.17 | 260.6 ± 22.45 | < 0.01 |
| Duration of the motor block | 183.3 ± 20.21 | 190.8 ± 39.74 | ns |
Abbreviation: min, minutes; ns, not significant.
aP < 0.01: highly significant; P < 0.05: significant.
5. Discussion
Pain is considered one factor of maternal morbidity not only in the postoperative period but also during the antenatal period in the form of pelvic pain. Although the pelvic pain present during the antenatal period is not harmful to either the mother or fetus, various measures have been described to make the gestational and puerpereal periods tolerable and satisfying to both the mother and family (13). Recent techniques for postoperative management in abdominal surgeries include ultrasound-guided TAP block, which has produced promising results (14). Similarly, TAP blocks are effectively used for post-caesarean analgesia (15). Providing high-quality analgesia is of paramount importance in developing countries and in all hospital settings. Therefore, intrathecal adjuvants are one of the easiest and most accessible methods of offering pain relief.
Midazolam is a relatively water-soluble benzodiazepine (16) and is extensively used in both critical care medicine and in the operating room for its sedative, anxiolytic, and amnesic effects (6). Another relatively newer concept for intrathecal midazolam in anesthesia practice is as an adjuvant. Midazolam exerts its analgesic activity through benzodiazepine receptors, which are distributed in the gray matter of the cervical, thoracic, lumbar, and sacral regions of the spinal cord; the highest densities of receptors were localized within lamina II of the dorsal horn (17). The segmental analgesia produced by intrathecal midazolam is mediated by the benzodiazepine GABA receptor complex, which is also involved in other benzodiazepine actions (18).
It has further been argued that intrathecal midazolam reduces excitatory GABA-mediated neurotransmission in interneurons, leading to a decrease in the excitability of spinal dorsal horn neurons (19). In animal studies, research has shown that intrathecal midazolam increases the pain threshold by binding to benzodiazepine receptors in the spinal cord (20-23). One study (24) reported that when 2 mg intrathecal midazolam were added to 1.5 mL of 5% lignocaine in women who underwent a caesarean section delivery, postoperative pain relief was evident. A similar result was shown by Tucker and colleagues (25). The first reports of spinal midazolam in the peer-reviewed literature were by Niv et al. (26), who showed a reduction in small afferent-evoked somatosympathetic reflexes in anesthetized dogs with no effect on the resting arterial blood pressure. On the basis of the appreciation of the role of GABA in regulating motor tone, Muller et al. (27) reported an antispasticity effect of intrathecal midazolam in unanesthetized, spinally catheterized cats but little effect on normal motor function.
In our study, postoperative analgesia was significantly better and longer in the midazolam group as demonstrated by its significantly longer time until the first request for analgesia and also the lower need for rescue analgesics. This finding was in accordance with the study of Kim and Lee (28), who reported that the addition of 1 or 2 mg of intrathecal midazolam prolonged the postoperative analgesic effect of bupivacaine by approximately 2 hours and 4.5 hours, respectively, compared with controls after hemorrhoidectomy and used fewer analgesics in the first 24 hours after surgery. The result suggested a dose-dependent effect of intrathecal midazolam. Similar results were reported by other studies (29, 30). Prakash et al. concluded that 2 mg intrathecal midazolam provided a moderate prolongation of postoperative analgesia in cesarean patients (11). Similar observations were reported by previous studies (12, 31). Other than cesarean patients, similar observations were also provided for patients undergoing orthopedic and other types of surgeries (29, 32).
The second observation in our study was that the median peak sensory level (T4) and motor block (bromoge 3) achieved with intrathecal midazolam were faster compared to the control group as reported by Sanwal et al. (31), who noted that decreasing the dose of bupivacaine to 7.5 mg along with 2 mg of midazolam did not affect the sensory level. Similar observations have been reported by other studies (33, 34). Further investigations have shown that the addition of midazolam or fentanyl to intrathecal bupivacaine does not alter the peak level of the sensory block (9, 35, 36). Similar observations were noted in our study.
The third observation that should be considered is the duration of the motor block, which was comparable in both groups (BC: 175.3 ± 20.21 vs. BM: 185.8 ± 39.74). This finding is consistent with the study of Shadangi et al. (37), whereas Bharti et al. (9) reported a prolonged motor block in their midazolam group. The result in our study was in accordance with Muller et al., who reported an antispasticity effect of intrathecal midazolam with little effect on normal motor function (27). The fourth observation in our study was the episodes of hypotension and the associated vasopressor requirement, which were significantly high in the control group compared to the midazolam group. This trend is consistent with studies by Sanwal et al. who reported that this relationship may be due to the bupivacaine-sparing effect of midazolam and concluded that intrathecal midazolam may allow the dose of bupivacaine to be reduced while still providing the same surgical anaesthesia with fewer episodes of bradycardia and hypotension (31). A similar observation was reported by previous studies (9, 25, 29). The Apgar score was comparable in both groups at 1, 5, and 10 minutes, which is consistent with the findings of previous studies (38).
Of note, the incidence of sedation was comparable in both groups. Patients in the midazolam group remained calm with a response to stimulus, which may be attributed to the anxiolytic effect of midazolam. Further, the incidence of nausea and vomiting was also found to be low in the midazolam group. In a study by Salami et al. (39), adjunct intrathecal midazolam was shown to potentially provide a more prolonged analgesia than opioids alone while also inhibiting their adverse effects, such as nausea and vomiting. A similar observation was reported by other studies (11, 12). It has been postulated that a possible mechanism for the anti-emetic effect of benzodiazepines could be an action at the chemoreceptor trigger zone, which reduce the synthesis, release, and postsynaptic effect of dopamine (40). The molecular basis of the specific antiemetic activity of intrathecal midazolam remains to be elucidated.
As noted in our study, shivering was decreased in the midazolam group. The mechanism for this is unclear and has to be determined. Various drugs like tramadol, dexmedetomedine, and magnesium sulfate have been added intrathecally to reduce shivering in caesarean patients (41-44). Further studies are recommended on the anti-shivering effect of midazolam.
5.1. Conclusions
Intrathecal midazolam provides significant and effective postoperative analgesia along with stable intraoperative hemodynamics without affecting the level of sensory and motor blocks. Adequate postoperative analgesia can be achieved with minimal side effects using intrathecal midazolam in PIH patients.