1. Background
Endoscopic retrograde cholangiopancreatography (ERCP) is a diagnostic and therapeutic procedure for the biliary tract and pancreas, which is done by endoscopy after injecting contrast dye through the duodenal papilla. Certain painful procedures may also be performed during ERCP, such as stenting, stone removal, visualization of the pancreatobiliary tract, laser lithotripsy, and sphincterotomy (1-4). Therefore, this procedure should be carried out under general anesthesia or deep sedation for immobility, analgesia, and patient comfort. ERCP complications can be related to sedation, such as arrhythmia, hypoxia, hypotension, and hypoventilation. The main challenges for the anesthesiologist during ERCP are apnea and airway obstruction, particularly with the patient in the prone position (due to the unavailability of the airways) (2, 3, 5, 6).
To assess the depth of anesthesia outside the operating room procedures, the Ramsay sedation scale was used (Table 1) (7).
| Clinical Score | Patient Characteristics |
|---|---|
| 1 | Awake; agitated or restless or both |
| 2 | Awake; cooperative, oriented, and tranquil |
| 3 | Awake but responds to commands only |
| 4 | Asleep; brisk response to light glabellar tap or loud auditory stimulus |
| 5 | Asleep; sluggish response to light glabellar tap or loud auditory stimulus |
| 6 | Asleep; no response to glabellar tap or loud auditory stimulus |
Scoring systems to determine the safest time to discharge patients after general anesthesia have been created, including the Aldrete scoring system (Table 2), on which a score of > 9 is suggested before discharge from the recovery unit (8).
| Clinical Parameter | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Activity: Able to move voluntarily or on command | 0 extremities | 2 extremities | 4 extremities |
| Respiration | Apneic | Dyspnea, shallow or limited breathing | Able to deep-breathe and cough freely |
| Circulation | BP ± 50 mm of pre-anesthesia level | BP ± 20 - 50 mm of pre-anesthesia level | BP ± 20 mm of pre-anesthetic level |
| Consciousness | Not responding | Arousable on calling | Fully awake |
| O2 saturation | O2 saturation < 90% even with O2 supplementation | Needs O2 inhalation to maintain O2 saturation > 90% | Able to maintain O2 saturation > 92% on room air |
Abbreviation: BP, blood pressure.
Midazolam is used for sedation in these procedures. It has sedative, amnesic, and anti-anxiety effects, but no analgesic effect (1, 9). Propofol is a lipophilic intravenous short-acting anesthetic drug that is most widely used in ERCP. Propofol has sedative and amnesic effects, but no analgesic effect (1, 6, 9). Administering high doses of propofol to deepen the anesthesia leads to dangerous cardiopulmonary side effects. Therefore, adding low doses of other drugs, such as ketamine and fentanyl, is recommended (1, 3, 9).
Fentanyl is a synthetic narcotic that is often used with midazolam for sedation and analgesia in ERCP. The most important complication of fentanyl is respiratory depression, which is intensified with concomitant administration of midazolam and will be more likely to require airway intervention. Another common side effect of the combination of midazolam and fentanyl for sedation is hypotension. The risk factors of these complications include old age, underlying disease (especially pulmonary disease), dementia, anemia, and obesity (1, 6, 9).
Ketamine is a non-barbiturate short-acting intravenous anesthetic drug that causes dissociative anesthesia. Low doses (less than 0.5 mg/kg) have acceptable analgesic and hypnotic effects, but also cause lower respiratory depression and fewer cardiac complications (3, 6, 7, 10-14).
Today, propofol and fentanyl are used during ERCP to achieve sedation and analgesia. These drugs are associated with complications, such as hypotension, respiratory depression, arterial oxygen desaturation, bradycardia, nausea, and vomiting (1, 14-16). To prevent or reduce these complications, other analgesic medications can be used in place of fentanyl (2, 3, 16). Studies have shown that the combination of ketamine and propofol can effectively prevent deep sedation of patients and cause lower postoperative nausea and vomiting, more stable hemodynamics, and a shorter discharge time after ERCP (11, 12, 16-18).
2. Objectives
The aim of this study was to compare the sedative and analgesic effects of fentanyl-propofol and propofol-ketamine.
3. Methods
This double-blind clinical trial was performed on 72 patients, ASA class 1 and 2, aged 30 - 70 years old, who were referred to the Ayatollah Rouhani hospital of Babol for elective ERCP. The sample size was calculated with a 95% confidence level, 80% power, and assuming Q1 = Q2 = 0.6 in terms of the Ramsay sedation scale, to find a 0.5 unit difference between the two groups. Each group required 23 samples, and 30 samples were included in each group to raise the test power.
The exclusion criteria were as follows: neurological, mental, pulmonary, or heart disorders; a short, thick neck; liver disease (Child-Pugh classification C); a history of gastrointestinal surgery; addiction; ASA class 3 or 4; pregnancy; known hypersensitivity to any of the study medications; and acute gastrointestinal bleeding. The research procedure was approved by the ethics committee of Babol University of Medical Sciences No. 4709 and registered in the Iranian registry of clinical trials (IRCT201410187752N6). The patients were given the necessary explanations, consent was obtained from each patient, and their data were kept confidential. All ERCPs were performed by a single experienced endoscopist (10 years’ experience with ERCP) and an anesthesiologist.
Syringes containing fentanyl and ketamine had the same shape (10 mL) and were coded by the anesthetist’s nurse. The patients, the anesthesiologist, the endoscopist, and the patient evaluator were unaware of the medication regimen.
After initial assessment and recording of standard monitoring, including electrocardiography, noninvasive blood pressure, and pulse oximeter, the patients were allocated into groups before the start of anesthesia. After measuring blood pressure, heart rate, and arterial oxygen saturation, 0.5 - 1 mg of midazolam was administered to each patient before ERCP. They were then consecutively divided into two groups: PK and PF. The intervention group received 0.5 mg/kg of ketamine and the control group received 50 to 100 mcg of fentanyl. All patients received propofol 0.5 mg/kg in a loading dose, followed by 75 mcg/kg/min in an infusion. If the Ramsay Sedation Scale score was < 2, propofol (20 mg) was administered as a rescue dose. Arterial oxygen saturation, respiratory rate, heart rate, blood pressure, and sedation were recorded based on the Ramsay sedation scale (Table 1) every 2 - 10 minutes, then every 5 minutes until the end of ERCP (7).
During ERCP, oxygen was delivered via nasal cannula. In case of loss of consciousness (based on the Ramsay scale), respiratory depression, and Sp02 of < 90%, or the lack of respiratory effort for more than 10 seconds, the drug infusion was discontinued and jaw-thrust maneuver and ventilation masks were initiated. If the apnea persisted despite intervention, tracheal intubation was performed and the patient was excluded from the study. The quality of analgesia based on a VAS scale of zero = no pain and 10 = the worst pain (Figure 1) was evaluated before discharge (6).
Endoscopist and patient satisfaction levels were determined based on a Likert scale from 0 - 10 (Figure 2) (9).
ERCP duration and the rescue dose of propofol were also recorded. Patient recovery time (based on an Aldrete score of > 9) were recorded (Table 2). The collected data were analyzed with SPSS version 22 statistical software.
The two groups were compared in terms of blood pressure, respiratory rate, heart rate, arterial oxygen saturation, endoscopist and patient satisfaction, pain score, rescue dose of propofol, and sedation scores based on the t-test and the P-value. The Chi-square test was used to compare the incidence.
4. Results
The study included 72 patients, 30 in the PK group and 42 in PF group. In terms of age, sex, and clinical parameters, the two groups showed no significant differences (Table 3).
| Group | PK (N = 30) | PF (N = 42) | P Value |
|---|---|---|---|
| Age, y | 56 ± 19.75 | 60.50 ± 15.66 | 0.119 |
| Sex | 0.719 | ||
| Male | 13 | 20 | |
| Female | 17 | 22 | |
| Basic MAP, mmHg | 90.75 ± 27.613 | 94.84 ± 19.263 | 0.505 |
| Basic respiratory rate, min | 12.31 ± 0.780 | 12.30 ± 0.988 | 0.964 |
| Basic SaO2, % | 97.33 ± 2.52 | 97.70 ± 1.87 | 0.709 |
| Basic heart rate, min | 83.8 ± 15.147 | 85.37 ± 15.199 | 0.699 |
Abbreviations: PK, propofol-ketamine; PF, propofol-fentanyl; MAP, mean arterial pressure; SaO2, saturation of arterial oxygen.
aP value based on t-test.
According to Table 4, the average sedation at 4 minutes in the PK group was higher than in the PF group (P = 0.037), but lower at 15 minutes (P = 0.035). There was no significant difference in the recovery time (P > 0.05).
| Time, min | 0 | 2 | 4 | 6 | 8 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|---|
| PK | 4.18 ± 0.82 | 4.28 ± 0.52 | 4.43 ± 0.57 | 4.60 ± 0.57 | 4.55 ± 0.51 | 4.31 ± 0.54 | 4.20 ± 0.42 | 4.20 ± 0.83 |
| PF | 4.17 ± 0.52 | 4.54 ± 0.55 | 4.17 ± 0.45 | 4.28 ± 0.39 | 4.74 ± 0.54 | 4.59 ± 0.47 | 4.71 ± 0.48 | 4.80 ± 0.44 |
| P Value | 0.970 | 0.054 | 0.037 | 0.114 | 0.224 | 0.164 | 0.035 | 0.195 |
Abbreviations: SD, standard deviation; PK, propofol-ketamine; PF, propofol-fentanyl.
aP value based on t-test.
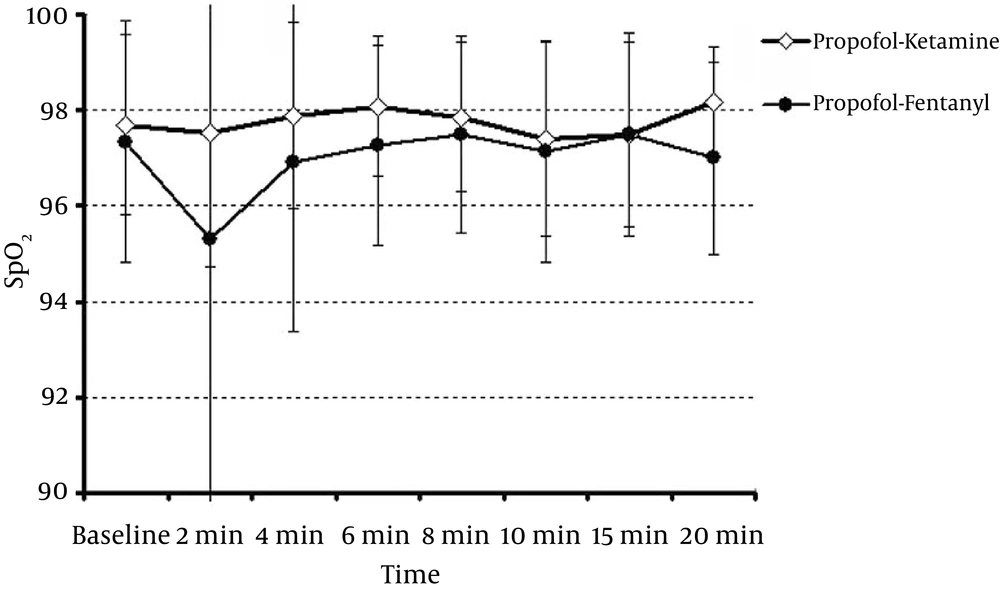
The mean arterial oxygen saturation in the PK and PF groups are presented in Figure 3. Arterial oxygen saturation showed no significant difference between the two groups. The mean respiratory rate in the PK and PF groups showed no significant differences at 0, 2, 4, 6, 8, 10, 15, and 20 minutes (P > 0.05).
In the PF group, seven patients (16.7%) had apnea, while three had no response to the PPV and were intubated; in the PK group, one patient (3.3%) had apnea and responded to PPV (P = 0.128).
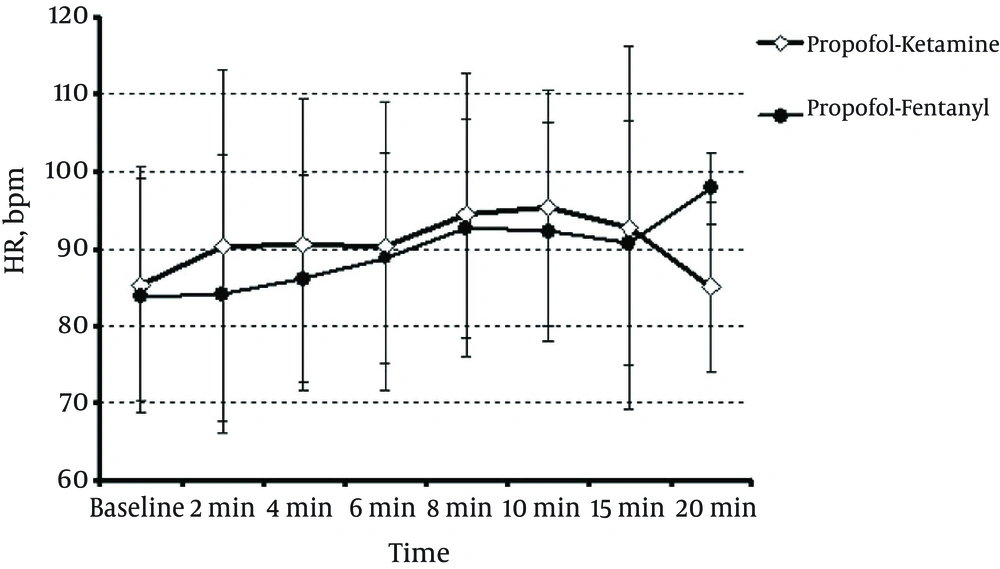
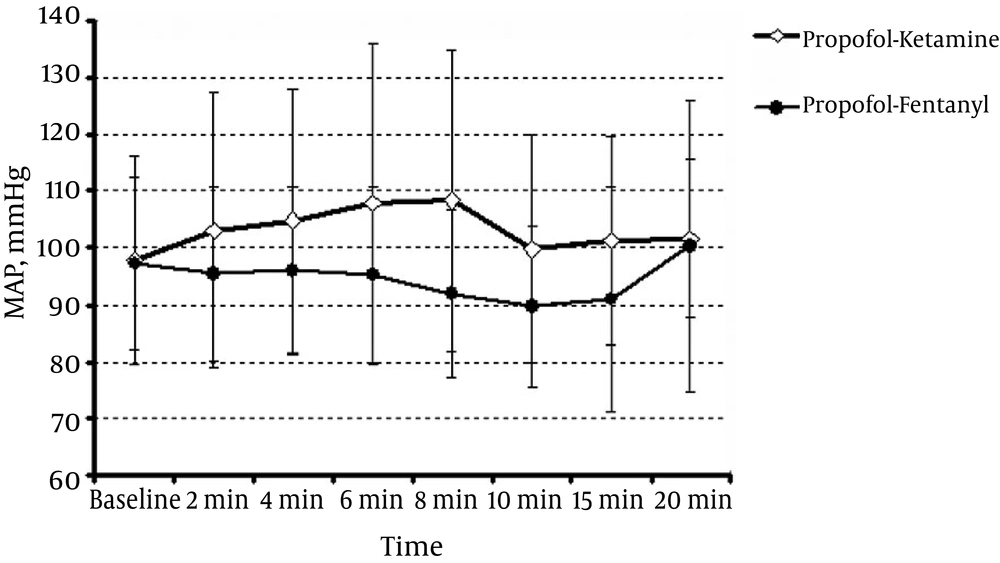
The mean heart rate in patients in the PK and PF groups (Figure 4) showed no significant difference (P > 0.05). Figure 5 shows the mean arterial pressure (MAP) in the two groups. At 8 min, the PK group was significantly different from the PF group (P = 0.021), but at other times, there was no significant difference (P > 0.05).
The pain scores were higher in the PK group. There was no difference in the two groups with regard to endoscopist and patient satisfaction. The mean rescue dose of propofol in both groups showed no significant difference (Table 5).
| Groups | PK (N = 30) | PF (N = 42) | P Value |
|---|---|---|---|
| Rescue dose, mg | 41.00 ± 64.71 | 30.95 ± 47.77 | 0.451 |
| Procedure time, min | 11.33 ± 7.308 | 8.93 ± 6.146 | 0.135 |
| Patient satisfaction | 7.45 ± 2.785 | 7.46 ± 2.937 | 0.646 |
| Endoscopist satisfaction | 7.91 ± 1.242 | 7.93 ± 1.118 | 0.317 |
| Post-procedural VAS | 1.97 ± 1.742 | 1.26 ± 0.921 | 0.037 |
| Recovery time | 14.17 ± 4.564 | 12.86 ± 3.339 | 0.164 |
Abbreviations: VAS, visual analogue scale; PK, propofol-ketamine; PF, propofolfentanyl.
aP value based on t-test.
Four patients in the PF group and one patient in the PK group had nausea and vomiting, which was not statistically significant (P = 0.257). Based on the Aldrete score, recovery times in the PK and PF groups were not significantly different (P = 0.164) (Table 5). In terms of the total duration of ERCP, there was no significant difference in the PK and PF groups (P = 0.135) (Table 5).
5. Discussion
The results showed that sedation was equal in both groups, but post-procedure pain in the propofol-fentanyl group was less than in the propofol-ketamine group. Patient and endoscopist satisfaction and recovery time showed no differences between the two groups. Hasanein et al. showed that the combination of ketamine and propofol resulted in better sedation quality than that of fentanyl and propofol, as well as fewer complications, in a study of obese patients undergoing ERCP (2).
In our study, considering the low dose of 0.5 - 1 mg of midazolam administered to both groups, the sedation level was acceptable and equal between the groups, but the frequency of respiratory complications in the ketamine/propofol group was lower. However, the results were not significant.
Aydoghan et al. studied the combination of propofol and ketamine compared to propofol alone in 100 patients undergoing upper gastrointestinal endoscopy. They concluded that the combination of propofol and ketamine causes shorter recovery time, better hemodynamic stability, and higher satisfaction than propofol alone (16).
Ramkiran et al. examined the effect of the depth of anesthesia using combinations of propofol-ketamine and propofol-dexmedetomidine by BIS in 70 patients who were candidates for ERCP. They concluded that the combination of propofol and low-dose ketamine led to less consumption of propofol, faster recovery, and more favorable hemodynamic effects compared to propofol-dexmedetomidine (19).
Tosun et al. conducted a study on upper GI endoscopy in children, using propofol-ketamine and propofol-fentanyl combinations. The results showed that propofol-ketamine caused deeper sedation (20).
Fabbri et al. compared the combination of ketamine–propofol and low-dose remifentanil with low-dose remifentanil/propofol in ERCP. The results showed that the combination of ketamine/propofol and low-dose remifentanil was more effective at preventing deep sedation, with a shorter recovery time (11).
Arora, in a review study on a combination of propofol and ketamine (ketofol) in a bolus dose during emergency procedures, concluded that ketofol caused safer and more effective sedation for such procedures (12).
In another study on ketofol used for the emergency induction of anesthesia in critically ill patients, Smischney concluded that this combination caused more stable hemodynamics (17). In our study, the mean blood pressure was similar except at the eighth minute.
In our study, the mean rescue dose of propofol during ERCP in the two groups was not significant.
In the propofol-fentanyl group of the present study, seven patients (16.7%) had apnea and three were intubated; however, in the propofol-ketamine group, only one patient (3.3%) had apnea, which did not require intubation. However, due to the small sample size, this difference was not statistically significant. The higher rate of respiratory complications was similar to that of other studies.
The frequency of nausea and vomiting in the present study was four cases in the propofol-fentanyl group and one case in the propofol-ketamine group.
Recovery time and endoscopist and patient satisfaction showed no significant differences between the two groups. The changes in arterial blood oxygen saturation, respiratory rate, and heart rate showed no significant differences between the two groups. The MAP was higher in the propofol-ketamine group than in the propofol-fentanyl group only at the eighth minute during ERCP (P = 0.021).
In previous studies on ketamine alone or in combination with other medications, this drug prevented deep sedation, and recovery time was shorter (21-24). In our study, however, the recovery time showed no difference between the two groups. This may be due to adding a low dose of midazolam (0.5 - 1 mg) to both groups.
The mean procedure time in the PK group in Abdalla et al.’s study was 24.5 minutes (3). In the present study, the mean procedure time was 11.33 minutes in the PK group and 8.93 minutes in the PF group, as all ERCPs were performed by one experienced endoscopist who had ten years’ experience in ERCP.
Post-procedure pain was lower in the PK group compared to the PF group, but endoscopist and patient satisfaction was similar in both groups. Other parameters, such as nausea, vomiting, and recovery time, were similar to previous studies.
5.1. Conclusion
The sedative effects of propofol-fentanyl and propofol-ketamine were acceptable and equal. Pain after ERCP in the PF group was less than in the PK group. The frequency of apnea was higher in the PF group, but not significantly. Patient and endoscopist satisfaction and recovery time showed no differences between the two groups. Patients at risk of respiratory depression are recommended to receive a combination of propofol and ketamine.
5.2. Limitations and Future Studies
Although the frequency of some complications, such as bradycardia, hypotension, and respiratory depression, were significantly higher in the PK group than in the PF group, the small sample size was a limitation against showing statistically significant differences. Therefore, it is recommended to conduct studies with larger sample sizes, to choose patients from a narrower age range, and to use different doses of the drugs.