1. Background
Extraperitoneal laparoscopy has become a common technique for many surgical procedures, especially inguinal hernia surgery. In this setting, it has been shown that laparoscopic technique has less chronic postoperative pain and numbness, fast return to normal activities and decreased incidence of wound infection and hematoma (1). We observed in our clinical practice that the advent of laparoscopy has resulted in extended indications, as in elderly patients with respiratory disease. Investigations of physiological changes occurring during extraperitoneal carbon dioxide (CO2) insufflation mostly focused on blood gas changes. Extraperitoneal insufflation has been reported to increase arterial pCO2, with data suggesting a more rapid and greater total increase in End-tidal CO2 (ETCO2) during extraperitoneal insufflation than pneumoperitoneum (2-4). To date, the impact of extraperitoneal CO2 insufflation on respiratory mechanics remains unknown, whereas changes in respiratory mechanics have been extensively studied in intraperitoneal insufflation (5-7).
2. Objectives
The purpose of our study was to investigate the effects of extraperitoneal CO2 insufflation on respiratory mechanics using two bedside techniques, the electrical impedance tomography (EIT) and wash-out/wash-in technique.
3. Patients and Methods
The study was approved by the Ethics Committee of the Sainte Anne military teaching Hospital. A written informed consent was obtained from patients. Patients aged 18 years and older scheduled for extraperitoneal laparoscopic surgery were recruited from October to November 2013. Patients with a body mass index > 35 kg/m², preexisting cardiac disease or pathologic lung function requiring noninvasive ventilation or oxygen therapy were excluded. Anesthetic management was standardized as follows. On their arrival in the operating room, standard monitoring device (electrocardiogram, pulse oximeter, and noninvasive arterial pressure [Intellivue MP40 monitor, Philips]) was applied. The level of anesthesia was monitored by bispectral index monitoring. Patients were given a 10 mL/kg of crystalloid solution intravenously before the induction. Preoxygenation was performed in pressure support ventilation (Inspiratory pressure of 8 cmH20, and PEEP of 4 cmH20). Anesthesia was induced and maintained with propofol, remifentanil or sufentanil and atracurium. Muscle relaxation was controlled by monitoring the TOF ratio. After induction, the trachea was intubated. Immediately after intubation, patients were mechanically ventilated (Engstrom Carestation ventilator; Datex-Ohmeda, General Electric Healthcare) in volume-controlled mode with a tidal volume (TV) of 8 mL/kg ideal body weight. The respiratory rate was adjusted to an end-tidal carbon dioxide concentration (EtCO2) between 35 and 40 mmHg. The inspiratory flow was settled to an inspiratory/expiratory ratio of ½. No PEEP was initially added. The inspiratory oxygen fraction (FiO2) was 0.5. An inspiratory pause of half a second was settled to monitor plateau pressure. Absence of intrinsic PEEP was evaluated by means of end-expiratory occlusion. Extraperitoneal insufflation was generated by insufflating carbon dioxide with pressure maintained at 10 mmHg.
3.1. Study Protocol
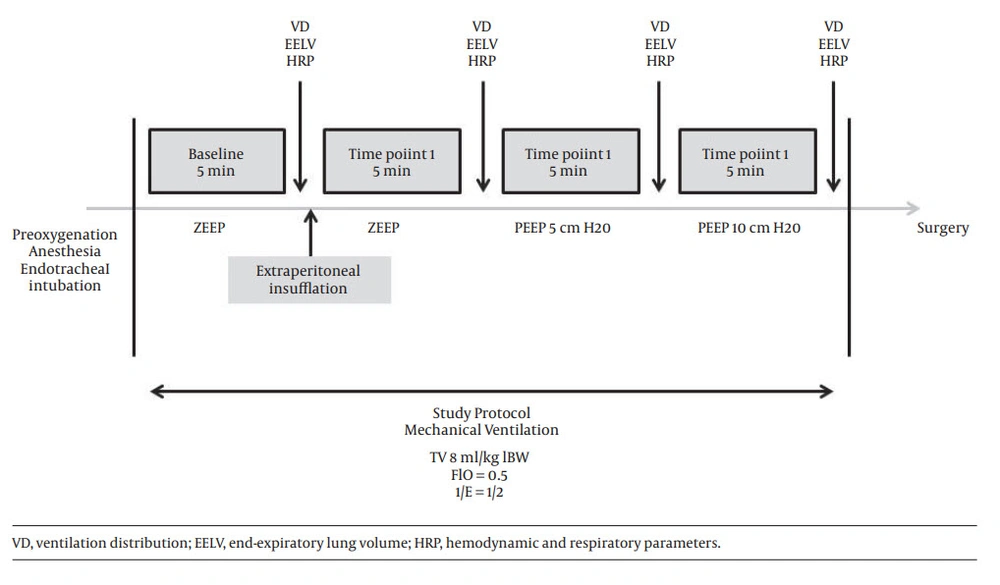
A schema of the protocol is provided in Figure 1. Anesthesia induction and study procedures were performed in supine position. Measurements were performed during baseline conditions and at three time points as described below:
-baseline conditions: after induction, patient was intubated and mechanically ventilated. Tidal volume was 8 mL/kg ideal body weight, respiratory rate was adjusted to EtCO2 value between 35 and 40 mmHg; FIO2 was 50%. No PEEP was added;
-time point 1: 5 minutes after extraperitoneal insufflation with no PEEP;
-time point 2: during insufflation, 5 minutes after application of a PEEP 5 cmH20;
-time point 3: during insufflation, 5 minutes after application of a PEEP 10 cmH20.
At baseline and at each measurement time point, heart rate, systolic and diastolic arterial pressure, EtCO2 value, BIS index, peak airway pressure and plateau pressure were recorded.
3.2. Ventilation Distribution Measurements
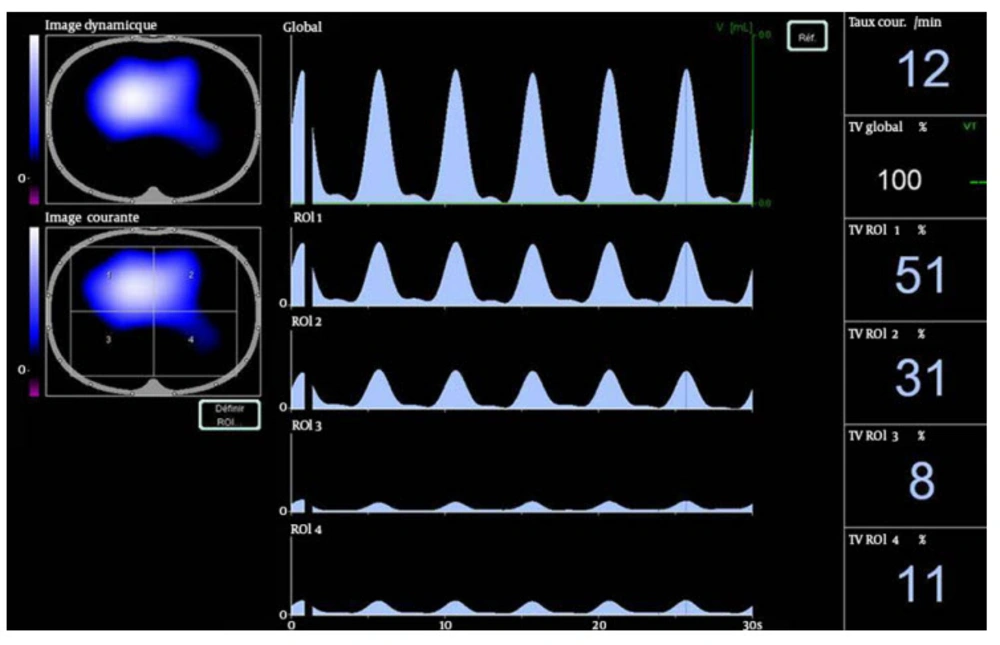
Ventilation distribution was assessed by EIT (Figure 2). EIT uses the electrical conductivity of chest to generate cross sectional images of lung inferred from surface electrical measurements realized by a 16 electrodes belt placed to the skin. A few milliamperes current is applied across two electrodes; all other electrodes are used to measure resulting voltage. In biological tissue, conductivity varies between tissues depending on factors as air content. The changes in impedance are correlated to changes in air content. We used the Pulmovista® 500 tomograph (Drager medical) to perform EIT measurements. The electrodes belt was placed around the patients’ chest between the 4th and 6th intercostal space. The Pulmovista® 500 tomograph measures impedance changes referring to an initial reference data set in real time. It generates a cross sectional image of the lung that can be divided in four regions of interest (ROI), each covering 25% of the ventrodorsal diameter. Impedance changes can be analyzed by ROI. To evaluate ventilation distribution, the number calculated per ROI is the sum of impedance changes in this ROI in relation to the sum of impedance changes of the whole EIT image. For instance, a number of 51% in ROI 1 indicates that 51% of the tidal volume variation takes part in this ROI (Figure 2). An increase in the fractional tidal variation per ROI indicates a redistribution of ventilation toward this ROI. In our study, ROI 1 and 2 represented ventral lung areas; whereas, ROI 3 and 4 represented dorsal lung areas.
3.3. End-Expiratory Lung Volume Measurements
End-expiratory lung volume (EELV) was assessed by a built-in modified nitrogen wash-out/wash-in technique. It was measured twice using an automated procedure available on the ventilator (GE Healthcare). EELV measurements reflect the amount of gas in the lungs and require FIO2 step of 0.1 as previously described (8, 9).
3.4. Thoracopulmonary Compliance Measurements
Thoracopulmonary compliance was calculated as ΔPaw/TV, where ΔPaw is the difference between plateau pressure and end-expiratory airway pressure, and TV is the tidal volume.
3.5. Statistical Analyses
Statistical analysis was performed using XLSTATS software (Addinsoft, Paris, France). Descriptive measures were used to present patients’ characteristics. No a priori power analysis was conducted, because we did not know the exact effect size that we would see. EELV and respiratory pressure changes during the study were compared using the Friedman two-way analysis of variance test. Ventilation distribution between the left and right lungs was compared at baseline and time point 1 using a Mann and Whitney test. Ventilation distribution changes throughout the study were compared for left and right lungs using the Friedman two-way analysis of variance test. The Friedman two-way analysis of variance test was used to compare heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), EtCO2 and BIS index during the study. A P value < 0.05 was required to reject the null hypothesis.
4. Results
Nine consecutive adult patients undergoing laparoscopic hernia inguinal repair were enrolled. There were eight male and one female. The mean age was of 61 ± 22 years. The mean body mass index was 24.8 ± 3.6 kg/m2. Patients’ characteristics are provided in Table 1. No relevant clinical complications occurred during the operation. According to the protocol, respiratory rate, tidal volume and flow rate were not changed during the investigation.
| Results | |
|---|---|
| Gender | |
| Male | 8 |
| Female | 1 |
| Age, y | 61 ± 22 |
| Weight, kg | 74 ± 11 |
| Height, cm | 173 ± 10 |
| BMI, kg/m² | 24.8 ± 3.6 |
| ASA classification, no | |
| 1 | 2 |
| 2 | 6 |
| 3 | 1 |
| Patients comorbidities, No. | |
| Smoker | 3 |
| Arterial hypertension | 4 |
| Coronary disease | 1 |
| Laparoscopic surgery, No. | |
| Inguinal hernia repair | 9 |
aAll data are presented as Mean ± SD unless otherwise specified.
4.1. Ventilation Distribution at Baseline
At baseline conditions, tidal volume was mostly distributed to ventral lung regions (74.5% ± 11.7 in ventral regions versus 20.7% ± 11.3 in dorsal regions, P = 0.0004) (Table 2).
| Baseline | Time Point 1 | Time Point 2 | Time Point 3 | |
|---|---|---|---|---|
| ROI 1 et 2 ventral region, % | 74.5 ± 11.7 | 74.5 ± 14.3 | 72.4 ± 13.4 | 61.5 ± 19.5 |
| ROI 3 et 4 dorsal region, % | 20.7 ± 11.3 | 23.1 ± 13.8 | 24.5 ± 12.9 | 34.8 ± 18.9 |
aAll data are presented as Mean ± SD.
4.2. Effects of Extraperitoneal Insufflation
After insufflation, ventilation distribution was not modified (74.5% ± 14.3 in ventral regions at time point 1 versus 74.5% ± 11.7 at baseline, P = 0.9). The difference of ventilation distribution remained significant between the ventral and dorsal regions (74.5% ± 14.3 versus 23.2% ± 13.8 P = 0.0004). Extraperitoneal insufflation was associated with a significant decrease in EELV measured by nitrogen wash-out/wash-in technique from 2115 mL ± 635 to 1716 mL ± 444 (P = 0.0018) (Table 3), and a significant decrease in thoracopulmonary compliance from 49.5 ± 6.3 to 40.1 ± 5 mL/cmH20 (P = 0.002). Insufflation did not modify plateau pressure values (13.1 cmH20 ± 1.9 versus 10.6 ± 1.8, P = 0.35), nor peak pressure values (21.5 ± 2.5 versus 18.8 ± 1.8, P = 0.354).
4.3. Effects of PEEP Application
A PEEP 5 cmH2O did not change ventral shift of ventilation. A lower ventral shift of ventilation with a PEEP 10 cmH20 was observed (61.5% ± 19.5 at time point 3 versus 74.5 ± 14.3 at time point 1, P = 0.008) (Table 2). A PEEP 5 cmH20 did not significantly change EELV and thoracopulmonary compliance (Table 3). Compared with ZEEP after insufflation was induced, PEEP 10 cmH20 significantly increased EELV from 1716 mL ± 444,4 to 2253 mL ± 616 (P = 0.006), and thoracopulmonary compliance from 40.1 ± 5.1 to 46.8 ± 9.6 (P = 0.031).
4.4. Changes in Hemodynamic and Respiratory Parameters During the Study (Table 3)
EtCO2 was significantly increased at time point 3 versus baseline (38.7 ± 5 versus 33.5 ± 2.4, P = 0.008). Extraperitoneal insufflation was associated with a significant increase in SAP (137.7 ± 30 versus 109.9 ± 12, P = 0.014), but DAP did not change significantly (80.8 ± 22 versus 65 ± 9.9, P = 0.052). Heart rate did not change significantly throughout the study.
BIS index value at time point 3 was significantly lower that of baseline (31.6 ± 8 versus 41.2 ± 11.9, P = 0.014).
| Baseline | Time Pointe 1Insufflation PEEP 0 | Time Point 2 Insufflation PEEP 5 | Time Point 3 Insufflation PEEP 10 | |
|---|---|---|---|---|
| Peak airway pressure, cmH2O | 18.9 ± 1.8 | 21.5 ± 2.5 | 24.4 ± 2.2 c | 29.3 ± 2.7 c |
| Plateau pressure, cm H20 | 10.6 ± 1.8 | 13.1 ± 1.9 | 16.5 ± 1.7 c | 21.4 ± 2 c |
| Thoracopulmonary compliance, mL/cmH2O | 49.5 ± 6.3 | 40.1 ± 5 c | 45.6 ± 6.5 | 46.8 ± 9.7 d |
| EELV, mL | 2115 ± 635 | 1716 ± 444 c | 1898 ± 542 | 2253 ± 616 d |
| EtCO2, mmHg | 33.5 ± 2.4 | 34.2 ± 4.7 | 37 ± 5.7 | 38.7 ± 5.1 c |
| SAP, mmHg | 109 ± 12 | 136 ± 30 c | 136 ± 23 c | 134 ± 23 |
| DAP, mmHg | 65 ± 10 | 80 ± 22 | 78 ± 20 | 76 ± 11 |
| Heart rate, /min | 70 ± 10 | 68 ± 11 | 65 ± 9 | 64 ± 13 |
| BIS index | 41 ± 11 | 36 ± 9 | 36 ± 10 | 32 ± 8 c |
aAbbreviations: EELV, end expiratory lung volume; SAP, systolic arterial pressure; DAP, diastolic arterial pressure.
b All data are presented as Mean ± SD.
cversus baseline P < 0.05.
dversus time point 1 P < 0.05.
5. Discussion
The present study demonstrated that extraperitoneal CO2 insufflation was associated with a significant decrease in FRC and thoracopulmonary compliance. Mechanical ventilation and general anesthesia were associated with a ventral distribution of tidal volume. Application of a 10-cmH20 PEEP led to a significant increase in FRC, thoracopulmonary compliance and homogenization of tidal volume distribution. Extraperitoneal laparoscopy has become a common surgical procedure, especially to inguinal hernia surgery. It has been shown that laparoscopic technique had less chronic postoperative pain, fast return to normal activities and decreased incidence of wound infection and hematoma (1). The advent of laparoscopy has resulted in extended indications, as in elderly patients with cardiorespiratory disease. However, the investigations on the respiratory effects of CO2 insufflation mostly focused on intraperitoneal insufflation. In this setting, it has been demonstrated in a CT-scan study that pneumoperitoneum by increasing abdominal pressure induced a mechanical compression and a cranial shift of the diaphragm between 1 and 3 cm. Besides, this study showed that pneumoperitoneum induced a mean increase of atelectasis volume of 66% (7). These effects promote alveolar collapse and atelectasis, which worsens respiratory mechanics resulting in decreased end-expiratory lung volume and thoracopulmonary compliance (2, 5, 6, 10-12). Reduction of EELV has been confirmed by CT scan in healthy patients (13) and by wash-out/wash-in method in both healthy and obese patients (14). Investigations of physiological changes occurring during the period of extraperitoneal CO2 insufflation mostly focused on blood gas changes. Extraperitoneal insufflation has been reported to increase arterial pCO2, with data suggesting a more rapid and greater total increase in End-tidal CO2 (ETCO2) during extraperitoneal insufflation than pneumoperitoneum (7, 14). In this pilot study, we demonstrated that extraperitoneal insufflation worsened respiratory mechanics and decreased thoracopulmonary compliance and FRC. We may postulate that extraperitoneal insufflation effects are similar to pneumoperitoneum effects as increase of abdominal cavity pressure and cranial movement of diaphragm. CT-scan study performed during extraperitoneal insufflation would help understanding the mechanisms, as previously published in pneumoperitoneum (7). Moreover, it would be interesting to evaluate the magnitude of extraperitoneal insufflation respiratory effects by comparing respiratory mechanics during extraperitoneal and intraperitoneal insufflation. In mechanically ventilated patients, EIT study showed that ventilation remained distributed mainly to ventral region (14). Our results at baseline are concordant with these results. The misalignment of ventilation during anesthesia is probably due to atelectasis formation in dorsal lung areas. This concept has been described fifty years ago (15). It has been visualized more recently in the study of Andersson et al. which described dorsal atelectasis by CT scan in patients mechanically ventilated after several minutes of stable anesthesia (7). We can expect that extraperitoneal insufflation may also lead to such atelectasis as suggested by the decrease in EELV we observed. However, CT scan studies as performed by Anderson et al. would be interesting to visualize atelectasis formation after extraperitoneal insufflation (7). To date, the impact of extraperitoneal insufflation on ventilation distribution is not known. Recently, the effect of PEEP and intraperitoneal insufflation on regional ventilation during laparoscopic surgery was studied by EIT (14). This study showed that effects of pneumoperitoneum on ventilation distribution were depending on the application of PEEP or not before pneumoperitoneum induction. In the ZEEP group, the authors found a lower ventral shift of ventilation after pneumoperitoneum, whereas in 10 cmH20 PEEP group, they observed a higher ventral shift. In our study, we observed a dorsal shift of ventilation distribution after 10 cmH20 PEEP application. The ventral shift of ventilation during anesthesia is likely due to the occurrence of dorsal atelectasis. We may postulate that 10 cmH20 PEEP application led to alveolar recruitment and decreased dorsal atelectasis, leading to a higher amount of ventilation in dorsal zones. The increase in arterial pressure observed after insufflation is concordant with previously published data (2, 4). The raise of EtCO2 observed has also been described in other studies (4). Our study had several limitations. First, it was only a pilot study with a small number of patients. Clinical relevance of our results can be questioned. Second, EIT is a noninvasive, radiation-free tool to assess regional lung ventilation at the bedside and the operating room. It is able to detect dynamically regional changes of ventilation during mechanical ventilation (14, 16, 17). However, EIT is a focal monitoring of ventilation and not a global monitoring of ventilation. The results of impedance changes provided by the device concerned a cross sectional section of the lung, depending on the location of the belt. In our study, the results of impedance changes we published are reliable with lower lung regions, and not upper lung regions. It could be interesting to design the same study with two belts locations, in the upper and lower regions. Another limitation was the inability of EIT to perform measures when electrocauter is on, because device switched to safety mode. Third, our study protocol stopped 15 minutes after pneumoperitoneum induction and started before the operation. It is hard to postulate on the effect of extraperitoneal insufflation on ventilation distribution during the operation and after it. However, Karsten et al. studied the effects of pneumoperitoneum during the operation, and the shift in ventilation distribution observed at the beginning of insufflation remained constant throughout the procedure (14). Finally, changes in respiratory functions may be also influenced by preoperative positions. According to our protocol, measurements were performed in neutral dorsal decubitus. During laparoscopic surgical procedures, patients may be in slight head-down position, and pressure of abdominal contents on the diaphragm is likely to cause a higher decrease in thoracopulmonary compliance, and FRC. In conclusion, the current study showed that extraperitoneal insufflation worsens respiratory mechanics, as previously described in intraperitoneal insufflation. Application of 10 cmH20 PEEP increased FRC and led to homogenization of ventilation distribution. These preliminary results may justify other studies with a greater number of patients to evaluate the clinical impact of respiratory changes during CO2 extraperitoneal insufflation.