1. Background
Anesthesia induction in pediatric patients with a recent history of common cold is a serious clinical dilemma (1-3). Adverse respiratory events are evidently more common in this specific population. Many studies have been performed to reduce the risk of these complications among patients. Utilization of laryngeal mask airways (LMAs), instead of endotracheal tubes, or lidocaine administration (intravenous or topical administration on LMA) has shown significant improvements in the outcomes, while in pediatric patients without upper respiratory tract infections (URIs), the results have not been promising (4-6). Also, different anesthetic methods and medications have been continuously investigated to reduce the risks as much as possible (4).
2. Objectives
In the present study, we aimed to determine whether preoperative intravenous administration of corticosteroids would benefit the pediatric population. Therefore, the target population randomly received corticosteroids or placebo, and the adverse intra- and postoperative respiratory outcomes were evaluated.
3. Methods
3.1. Inclusion Criteria
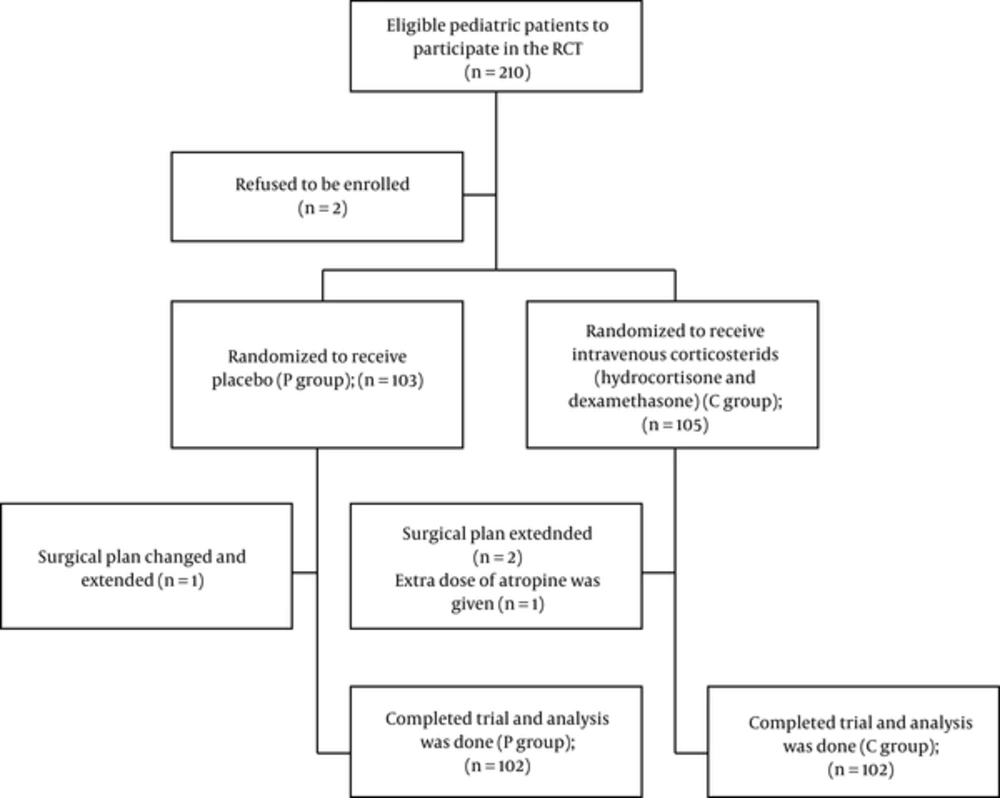
The ethics committee of Shahid Beheshti University of Medical Sciences approved this study (IRCT201307023436N2), and written informed consents were obtained from the guardians or parents. A total of 204 pediatric patients completed the trial (CONSORT flowchart presented in Figure 1). The present study was conducted from 08/03/2013 to 01/30/2014 at Labbafinejad hospital (a university-affiliated referral hospital), Tehran, Iran.
The inclusion criteria for pediatric patients were as follows: (1) age range of 1 - 6 years; (2) parents’ recognition of mild URI symptoms (eg, cough, sneeze, and nasal discharge or congestion), initiated within the past 2 weeks; (3) no evidence of the symptoms of bacterial (eg, ill appearance evaluated by the anesthesiologist, axillary temperature > 38°C, and purulent discharge or sputum) or lower respiratory tract infection (eg, crackles or wheezing sounds); and (4) any other medical condition (eg, respiratory conditions, cardiac diseases, allergies, conditions resulting in difficult airway management, and neurological disorders). All the patients had to be anesthetized for the ophthalmologic examination in a short period of time (less than 6 weeks) to prevent any deleterious outcomes (6, 7).
3.2. Exclusion Criteria
The exclusion criteria were as follows: (1) use of any medications not mentioned in the protocol; (2) experience of extended anesthesia (more than 1 hour); and (3) any changes in the surgery plan during the same session. The patients who met these criteria were excluded from the trial and registered in the CONSORT flowchart.
3.3. Preoperative Evaluation
URI was diagnosed based on a positive history of nasal congestion or discharge, initiated 2 weeks prior to surgery, as mentioned by the parents or guardians (6, 7). On the day of surgery, preanesthesia symptoms of common cold (eg, runny nose, nasal congestion, cough, sputum, and sneeze) were evaluated by an anesthesiologist (appendix 1 in the supplementary file) (6, 7). Patients who scored above 2 on any of the items in the questionnaire were considered to have moderate to severe URI and were not enrolled in the study. Also, passive smoking was recorded.
3.4. Randomization and Blinding
Eligible pediatric patients, whose parents had agreed to their participation, were randomized (using the of of random numbers) to receive either intravenous placebo (group P) or intravenous glucocorticoids (1 mg/kg of hydrocortisone; Exir Co., Iran and 0.1 mg/kg of dexamethasone; Caspian Tamin Pharmaceutical Co., Rasht, Iran) 10 minutes prior to anesthesia induction (group C).
In order to make this trail blinded, all trial medications (placebo and corticosteroids) were prepared in separate rooms using similar syringes, based on the patient’s weight. Then, they were given to the anaesthesiologist in charge to be administered.
3.5. Study Protocol
The patients were placed on the operating and basic monitoring was applied and recorded. An intravenous line was placed, and both groups received a 2 cc syringe, containing either normal saline (group P) or glucocorticoids (group C). Anesthesia induction was achieved with incremental doses of sevoflurane (starting with a 3% concentration and increasing the dosage by 1% every 30 seconds as required), 50% nitrous oxide (N2O), and oxygen (50%). Also, atropine (0.01 mg/kg) and lidocaine (1.5 mg/kg of Lignodic; Caspian Tamin Pharmaceutical Co., Rasht, Iran) were injected for all the patients.
A lubricating gel was applied over the LMA (Well Lead Medical Co., Guangdong, China). In a deep state of anesthesia, marked by the cerebral state index (CSI) (Cerebral State Monitor, Danmeter-Goalwick Holdings Ltd., Odense, Denmark) in the range of 40-60, an end-expiratory sevoflurane concentration of at least 2.1% was administered (with regular breathing), and LMA was inserted by an anesthesiologist. LMA size was selected based on the manufacturer's instructions.
Sevoflurane (2% - 3%) in oxygen and N2O (50% and 50%, respectively) was utilized to maintain anesthesia. The patients were planned to breathe spontaneously; therefore, muscle relaxation was not applied. At the end of the surgery, sevoflurane and N2O were discontinued and the patient only received 100% oxygen; LMA was removed under deep anesthesia. The anesthesiologist decided whether to suction the oral cavity on LMA or not. The patients breathed 100% oxygen with the face mask until they were fully awake (ie, opening the eyes or crying). Afterwards, they were positioned laterally and transferred to the recovery room, while receiving 3 - 5 L/min of oxygen with the face mask.
3.6. Outcome Assessment
Based on previous studies, coughing, given its higher incidence among adverse respiratory events, was measured as the primary outcome through direct observation during recovery and a telephone call on the first postoperative day (appendix 2 in the supplementary file) (6, 7).
The secondary outcomes were as follows (appendix 2 in the supplementary file): (1) bronchospasm, assessed by the anesthesiologist and characterized by inspiratory/expiratory wheezing sounds (6, 7); (2) apnea characterized by the absence of air flow for more than 10 seconds through the airway without any respiratory effort (6, 7); and (3) laryngospasm (partial) indicated by stridor sounds for more than 10 seconds (6, 7). Furthermore, desaturation and vomiting were scored and evaluated (6, 7). Any cardiovascular events or 7-day readmission due to respiratory problems were reported (6, 7).
3.7. Statistical Analysis
Confounding factors can greatly affect the incidence of respiratory events. Based on a pilot study, 25% of pediatric patients with URI (anesthetized without glucocorticoid) experienced postoperative cough. With respect to these findings and a 10% decline in postoperative cough with intravenous glucocorticoid (power of 80% and first order error of 0.05), a sample size of 100 patients was calculated for each group. Considering the possibility of dropout, a total of 210 patients were included.
We utilized SPSS version 16 to analyze the data. If the data were parametric and normally distributed, independent t test or Chi square was used. Wilcoxon or Mann-Whitney U test was applied for nonparametric parameters. The significance level was set at 0.05.
4. Results
A total of 210 subjects were considered eligible for the study; however, 204 patients completed the trial (Figure 1). All variables were normally distributed. The demographic data were not significantly different between the 2 groups (Table 1). Preoperative symptoms of patients are presented in Table 2. No significant difference was found between the 2 groups regarding preoperative symptoms, onset of common cold, or frequency of passive smoking.
Adverse respiratory events during anesthesia, recovery, and after discharge are presented in Table 3. The incidence of cough in the patients was not significantly different between the groups. Other variables including apnea, laryngospasm, bronchospasm, desaturation, vomiting, increased coughing on the first postoperative day, and 7-day readmission due to respiratory events were also not significantly different between the groups. Also, cardiovascular events (eg, cardiac arrest, bradycardia, arrhythmia, or hypotension requiring medication) did not occur in any of the patients.
| Variables | Group C (n = 102) | Group P (n = 102) | P Value |
|---|---|---|---|
| Cough | NS | ||
| No | 70 | 67 | |
| Yes (but not troublesome) | 30 | 34 | |
| Yes (with desaturation) | 2 | 1 | |
| Laryngospasm | NS | ||
| No | 88 | 86 | |
| Yes | 14 | 16 | |
| Bronchospasm | NS | ||
| No | 55 | 95 | |
| Yes (inspiratory wheezing) | |||
| Yes (inspiratory and expiratory wheezing) | 2 | 1 | |
| Desaturation | NS | ||
| SpO2 > 95% | 88 | 97 | |
| SpO2 of 90% - 95% and spontaneous resolution | 4 | 5 | |
| Apnea | NS | ||
| No | 93 | 97 | |
| Yes | 9 | 5 | |
| Vomiting | 0.3 | ||
| No | 98 | 96 | |
| Yes (once) | 4 | 6 | |
| Cardiovascular events | 0 | 0 | |
| Increased coughing on the first postoperative day | 8 | 7 | |
| Respiratory readmission | 0 | 0 |
The Incidence of Adverse Perioperative Eventsa
5. Discussion
The present study revealed that intravenous corticosteroids (a combination of hydrocortisone and dexamethasone) have no significant effects on respiratory outcomes in pediatric patients with URI, undergoing LMA anesthesia. Recruitment of a larger sample size may result in a significant difference between the groups. However, we believe that corticosteroid administration for achieving differences below 10% in respiratory complications (assumption for sample size calculation) has no clinical justification, and the risk-benefit assessment may incline towards the side effects of corticosteroids.
There is controversy in the literature regarding the interval between the onset of URI and anesthesia induction, which can induce more adverse respiratory events. In this regard, Von-Ungern Sternberg et al. concluded that an interval of 2 weeks since the onset of common cold has the greatest impact. On the other hand, Tait et al. and some other researchers advocated a longer vulnerability period (4 - 6 weeks) for further respiratory complications (1, 3, 4, 6-10).
Use of LMA has been supported over endotracheal intubation and anesthesia face masks in pediatric patients with URI, undergoing ophthalmic examination under anesthesia (5, 6). In general, different factors can affect the outcomes. In the present study, we tried to reduce the confounding factors by limiting the age range of the patients (1 - 6 years), applying similar medications and protocols, and reducing the diversity of examination time (not more than 1 hour).
In the present study, we applied atropine for all the patients, as recommended by Von-Ungern Sternberg et al. to prevent bradycardia and decrease secretions in cases with URI (4). However, Tait et al. found no benefits for glycopyrrolate in this specific population (11). They administered intravenous lidocaine (similar to the present study) instead of topical lidocaine on LMA, as they had found more positive effects for the intravenous approach in their previous study (7). Overall, there is a great body of evidence supporting the anti-inflammatory and analgesic effects of lidocaine, which may lead to a decline in adverse respiratory consequences (9, 12-18).
Varying incidence rates have been reported for the associated adverse events. In a review by Orliaguet et al., the incidence of laryngospasm ranged between 1/1000 and 20/100 (2). This study perhaps included a general pediatric population (with and without URI), undergoing anesthesia. On the other hand, Schebesta et al. reported an incidence rate of 41% for intraoperative spasms (bronchospasm and laryngospasm) in pediatric patients with URI, who did not receive lidocaine; however, the incidence rate decreased to 18% by using lidocaine on LMA (9).
In the present study, laryngospasm occurred in about 14% of the intravenous corticosteroid group and 16% of the placebo group. The similarity in the incidence of laryngospasm (as well as many other outcomes) in the study groups is probably related to the fact that we have reached a point (application of LMA, lidocaine, and atropine, as well as expert treatment of pediatrics with URI) where a slight reduction in the incidence of respiratory events requires great efforts and large sample sizes.
Corticosteroids have been largely studied in pediatric patients with common cold, allergies, and rhinitis (19-23). A systematic Cochrane review on children and adults showed no benefits for intranasal corticosteroids in symptomatic reduction of common cold (22). However, Meade et al. reported a significant reduction in postextubation stridor in children who received corticosteroids (24).
In the mentioned study, the authors believed that corticosteroids (hydrocortisone for its rapid and short-acting effects and dexamethasone for its long-lasting subcellular binding) have no beneficial independent effects on URI for previously healthy children. However, in cases with allergic rhinitis and atopic or hypersensitive allergic airways (which are not rare), corticosteroids seem helpful. It also seems rational to apply corticosteroids in pediatric patients with URI and endotracheal tube insertion (eg, full-stomach emergencies).
In conclusion, we believe that the virus type, as well as the individual’s inflammatory responses, has inevitable impacts on the respiratory outcomes. Therefore, further studied should be designed to evaluate the molecular and cellular aspects of respiratory events in vulnerable populations.
5.1. Conclusion
Based on the present findings, intravenous injection of corticosteroids has no beneficial effects for pediatric patients with minor uncomplicated URI (without a history of allergies), undergoing LMA anesthesia.