1. Background
Angelman syndrome is a rare genetic disorder with prevalence of approximately 1:52,000 live births (1). It is characterized by severe developmental disability, microcephaly, ataxia, seizures, sleep disturbances, and idiosyncratic movements and behaviors (e.g., hand-flapping, tongue-thrusting, and frequent laughing and smiling) (2). Most commonly it is caused by a maternal deletion of the ubiquitin-protein ligase (UBE3A) gene at Prader-Willi Angelman syndrome critical region 15q11-q13 (3). This deletion also affects the gamma-amino butyric acid (GABA) (A) beta-3 receptor subunit gene (4) which contributes to the high rate of epilepsy encountered in these patients.
Because of their intellectual disability and behavioral abnormalities (anxiety), patients with Angelman syndrome may have difficulty with medical evaluations and may require general anesthetics for seemingly innocuous procedures such as dental care, (5) but they may also require anesthesia for larger procedures such as spine surgery to correct scoliosis (6). Due to the rarity of Angelman syndrome, there are no large studies to address whether these patients may have specific complications during anesthesia. There are several features of the syndrome that have theoretical implications for anesthetic management. For example, GABA (A) receptor abnormalities could affect responses to commonly used intravenous anesthetics. Both decreased and increased sensitivity to anesthetic agents have been reported (7, 8). Patients with Angelman syndrome also may have high vagal tone. There are reports of malignant bradydysrhythmias triggered by laughing episodes (9, 10). and profound bradycardias have occurred during anesthetics (7, 11). These patients may have craniofacial abnormalities and excessive drooling that could complicate airway management (7, 12). Lastly, these patients have a “happy” demeanor, which can confound pain severity assessment (13).
In order to better understand how this unique patient population tolerates perioperative management, we conducted a retrospective review of all patients with Angelman syndrome who underwent anesthetic management at our institution during the past decade and a half. We also conducted a systematic review of the literature and summarized the published perioperative experience with these patients.
2. Methods
This retrospective cohort study was approved by the Mayo clinic institutional review board (Rochester, MN). Consistent with Minnesota Statute 144.295, we included only patients whose legal guardian had provided authorization for research use of their medical records.
Subjects were identified as patients with Angelman syndrome who were treated in our institutional division for genetic disorders and had surgery from January 1st, 2000 to December 31st, 2016. We performed an in-depth review of the electronic health records of these subjects, searching for pertinent data regarding demographic information, preexisting comorbidities, anesthetic management, surgical procedures and complications occurring both intraoperatively and within 30 days of each surgical procedure. We directed specific attention to the anesthetic records seeking to identify any hemodynamic instability, bradycardia, idiosyncratic responses to hypnotics, airway management, postoperative seizure, and difficulty with postoperative pain assessment.
We developed a descriptive summary of all information related to the demographics of these subjects. We then analyzed the demographic, epidemiologic, perioperative complications, and other data for continuous variables, providing mean ± SD or median [interquartile range] as appropriate, and categorical variables as frequency percentages.
In order to review the current knowledge regarding anesthesia complications for patients with Angelman syndrome, we performed a comprehensive literature search of MEDLINE (1966-present), Scopus (1960-present), and Embase (1988 - present). Our search strategy included the use of the following and related terms: “Angelman”, “Angelman syndrome”, “Happy Puppet”, “anesthesia”, “anesthetic”, “nerve block”, “postoperative complications”, “intraoperative care”, “perioperative care”, “intraoperative complications”, and “surgery”.
3. Results
Six patients with Angelman syndrome were identified who received anesthetics during the study period. These patients underwent 18 procedures; 14 performed with general anesthesia, and 4 under monitored anesthesia care. Four patients (#1, 2, 5, 6) had maternally-derived deletion, one (#4) had abnormal deletion on the 15th chromosome compatible with Angelman syndrome, and another (#3) had a methylation imprinting defect without deletion of the maternally-derived Prader-Willi Angelman syndrome critical region. Demographic and clinical variables of these patients are presented in Table 1. Patients had severe developmental delay and were non-verbal except for Patient #3 who had a mild phenotypic Angelman syndrome variant manifested by modest developmental delay.
| Patient (No) Sex, Age at First Surgery | Intellectual Delay, Verbal Skills, Behaviors, Disordered Sleep | Seizures, Treatment | Movement Disorders | Craniofacial Abnormalities | Other |
|---|---|---|---|---|---|
| 1. male, 7 mo | Severe, nonverbal, excitable/laughing, disordered sleep | Staring episodes, 2 - 3 week levetiracetam | Tremulous limbs | Microcephaly, plagiocephaly, strabismus | GERD |
| 2. female, 2 y | Severe, nonverbal, excitable/laughing, disordered sleep | Abnormal electroencephalography, no seizures | Ataxia, tremulous limbs | Microcephaly, plagiocephaly, hypertelorism | |
| 3. female, 7 ya | Mild delay only | None | None | Frontonasal dysplasia | |
| 4. female, 16 y | Severe, nonverbal, excitable/laughing | Tonic-clonic, several mo Valproic acid, zonisamide, levetiracetam | Ataxia, tremulous limbs | Microcephaly, strabismus | |
| 5. male, 8 y | Severe, nonverbal, excitable/laughing, disordered sleep | Tonic-clonic, 2 - 3 mo Clobazam, valproic acid, topiramate | Ataxia, tremulous limbs | Strabismus | Scoliosis eosinophilic esophagitis |
| 6. male, 29 y | Severe, nonverbal, excitable/laughing, disordered sleep | Spastic seizures, well controlled Valproic acid | Wheelchair bound, tremulous limbs | Small head, hypertelorism, protruding tongue |
Abbreviations: GERD; gastroesophageal reflux disease; mo, month; y, year.
aMild presentation of Angelman syndrome secondary to methylation imprinting defect in chromosome 15q11.2-q13, this was not secondary to maternal deletion, but parents declined further genetic work up to determine precise genetic defect.
Procedural and anesthetic variables are summarized in Table 2. The clinical course for most cases was unremarkable, except for a few notable observations. Patient # 2 (2 year-old, 10 kg girl) developed severe bronchospasm which resulted in hypoxemia with an oxyhemoglobin saturation of 67% during an MRI scan under general anesthesia with endotracheal tube. The bronchospasm resolved with positive pressure ventilation and a propofol bolus. Patient #4 (16 year-old, 47 kg girl) had a prolonged emergence following extraction of retained deciduous teeth under general anesthesia with endotracheal tube. She had been administered 20 mg oral midazolam for preprocedural sedation and 50 mcg of fentanyl for intraoperative analgesia, and anesthesia was maintained with desflurane. Postoperatively, she was deeply sedated for approximately 2.5 hours, but had good respiratory drive. She was administered 0.1 mg flumazenil with prompt resolution of sedation and was subsequently extubated and discharged home. Lastly, Patient #6 (28 year-old, 78 kg man) was electively intubated with a videolaryngoscope out of concern for a potentially difficult airway. Of note, Patient #6 did experience a febrile seizure in the context of urosepsis antecedent to monitored anesthetic care for a diagnostic computer tomography scan.
| Patient (No), Age, Weight (kg) | Procedure Duration (min) | Anesthetic Typea | Analgesiab | PACU Duration (min) Disposition |
|---|---|---|---|---|
| 1 | ||||
| 7 mo, 7 | MLB, EGD, 78 | Sevo/Prop | - | 68, outpatient |
| 8 mo, 7 | MRI, 117 | Sevo/Prop | - | 47, outpatient |
| 2 | ||||
| 2 y, 10 | MRI, 111 | Sevo/Prop | - | 101, outpatient |
| 2 y, 11 | Strabismus, 141 | Sevo | Fent/APAP | 95, outpatient |
| 3 y, 12 | T/A, MLB, 93 | Sevo | NSAID | 44, outpatient |
| 3 | ||||
| 7 y, 33 | T/A, 58 | Sevo | Morphine, LA | 32, outpatient |
| 4 | ||||
| 16 y, 47 | Dental, 90 | Mid/Prop/Des | Fent/NSAID, LA | 234, outpatient |
| 5 | ||||
| 8 y, 33 | EGD, 51 | Mid/ Sevo/Prop** | - | 36, outpatient |
| 8 y, 33 | EGD, 35 | Mid/ Sevo/Prop** | - | 45, outpatient |
| 8 y, 33 | EGD, 34 | Mid/ Sevo/Prop** | - | 22, outpatient |
| 8 y, 31 | T/A, EGD, 111 | Sevo, Atracuriumc | Fent, LA | 36, ICUd |
| 9 y, 31 | EGD, 24 | Sevo** | - | 39, outpatient |
| 20 y, 106 | EGD, 34 | Sevo/Prop** | - | 14, outpatient |
| 6 | ||||
| 28 y, 78 | LP, 44 | Mid/Prop/Iso Succinylcholine | LA | 53, ward |
| 28 y, 78 | PICC, 65 | Mid/Prop†† | Fent, LA | 43, ward |
| 28 y, 78 | CT, 22 | Mid/Prop†† | - | 57, ward |
| 34 y, 89 | LP, 77 | Mid/Prop/Ket†† | Fent, LA | 12, ICUe |
| 34 y, 89 | CT, 39 | Mid/Prop†† | - | 116, ward |
Abbreviations: APAP, acetaminophen; CT, computerized tomography scan; Des, desflurane; EGD, esophagogastroduodenoscopy; Fent, fentanyl; ICU, intensive care unit; Iso, isoflurane; Ket, ketamine; LA, infiltration of procedure site with local anesthetic; LP, lumbar puncture; min , minute; mo, months; MLB, microlaryngoscopy and bronchoscopy; MRI, magnetic resonance imaging scan; Mid, midazolam; NSAID, nonsteroidal antiinflammatory drug; PICC, peripherally inserted central catheter; Prop, propofol; Sevo, sevoflurane; T/A, adenotonsillectomy; y, years.
aCases were performed under general endotracheal anesthesia unless noted with ** to indicate general mask anesthesia or †† to indicate monitored anesthesia care.
bNo patient was administered opioid or nonopioid analgesic during Phase I postanesthesia recovery.
cAtracurium 10 mg was administered with induction, but was not reversed.
dPlanned ICU admission for postoperative monitoring of airway effort.
eAdmitted to ICU for treatment of on-going sepsis that was unrelated to procedure.
The systematic review of the literature identified one case series of anesthetic management in English (14), one English case series of dental care which briefly discusses anesthetic management (15), a case series in German 13 and case reports in English (6-8, 11, 12, 16-21), Spanish (22-24), and Japanese (25-30). Two reports (8) appear to reference the same case.
4. Discussion
The most important observation in this case series is that surgical patients with Angelman syndrome generally tolerated anesthetic management well. Our systematic review of the literature found that even though the majority of anesthetics were unremarkable (6, 12, 14, 18-23, 25-27, 29, 30) there were periprocedural events which could be attributed to Angelman syndrome. These instances in context of specific components of Angelman syndrome are highlighted below and are summarized in Table 3.
| Feature | Implications |
|---|---|
| Intellectual disability | Require anesthesia for minor procedures |
| Nonverbal | Pain assessment can be confounded; parental input should be sought (13) |
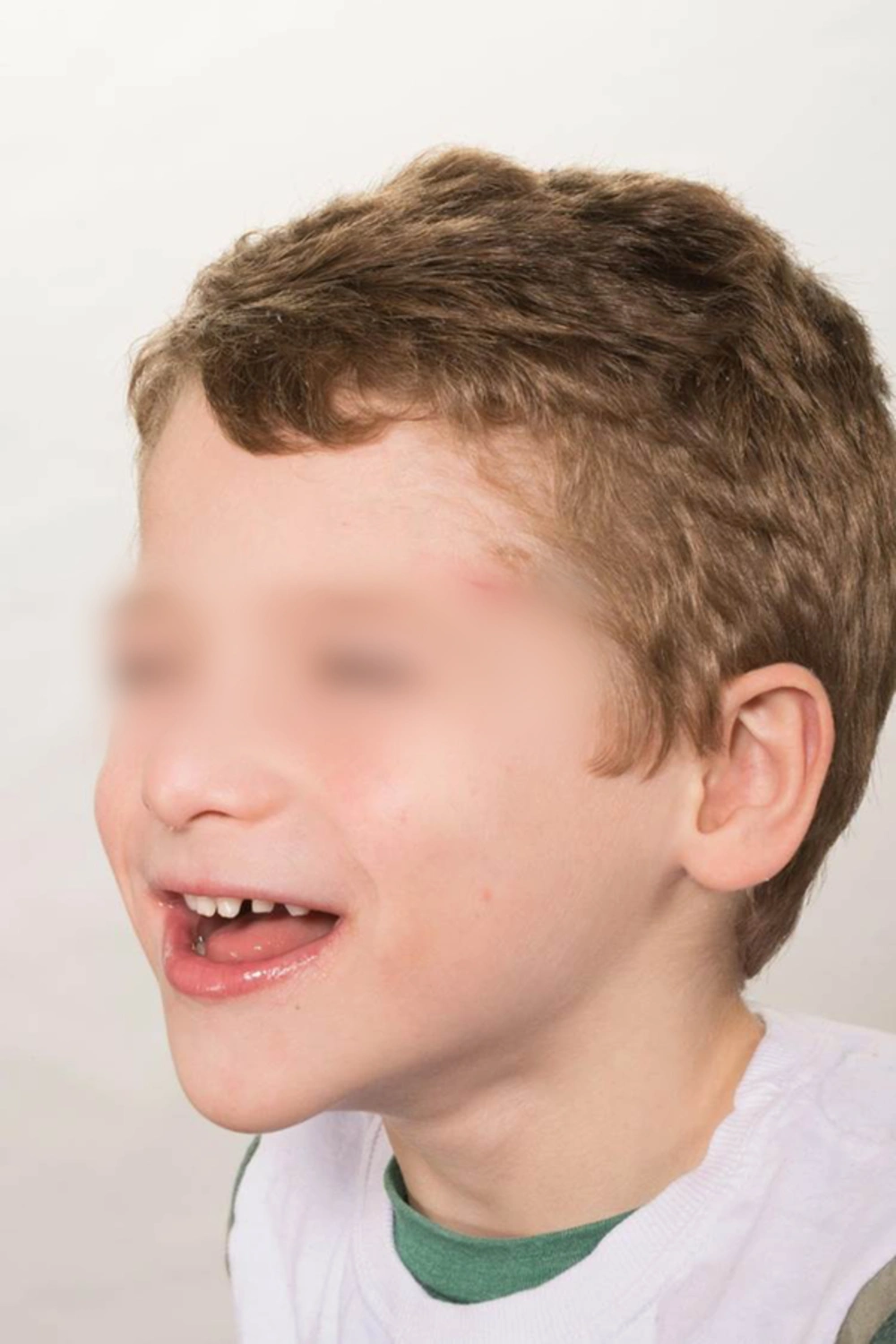
| Happy dispositiona | Reduced ability to assess the pain (Figure 1). |
| Disordered sleep | Monitor for postoperative sleep disturbances |
| GABA-A Abnormalities | Potential for increased/decreased sensitivity to benzodiazepines (Pt#4b) (8, 24) |
| Seizure disorders | Continue perioperative antiepileptic regimen |
| Monitor for increased seizure activity | |
| Craniofacial abnormalities | Potential for airway difficulty(Pt#6b) (7, 24, 28) |
| Increased vagal tone | Potential for profound bradycardia (7, 11) |
| Can be recalcitrant to anticholinergic medications | |
| Potential asystole during laughing outbursts (9, 10) | |
| Closely monitor heart rhythm during laparoscopic surgery | |
| Closely monitor all patients with previous vagal episodes | |
| Muscular abnormalities | Truncal hypotonia, ataxic gait, truncal hypertonia in adulthood |
| Potential implications for neuromuscular drug administration | |
| Respiratory abnormalities | Postoperative respiratory failure and disordered breathing patterns have been described (2, 14, 17) |
aUnprovoked episodes and outbursts of laughing, clapping hands, etc.
bIndicates patient and complication was described in this cohort.
4.1. Intellectual Disability
Patients with Angelman syndrome typically have profound intellectual disability and are often nonverbal. Even though patients with Angelman syndrome are usually good natured; the degree of cognitive impairment and limited communication skills limits their ability to cooperate during medical procedures. Therefore, they often require anesthetic even when undergoing minor procedures 5. In this series, Patients#4 and #6 were of the age that they should not have required anesthetic management if they had been of normal intelligence when they underwent simple procedures (dental extraction and minor diagnostic procedures). Placement of intravenous lines may be difficult because these patients are often uncooperative (17). Further, these patients typically appear to be happy and frequently laugh and smile, even under inappropriate circumstances (Figure 1). These characteristics could complicate assessment of pain postoperatively. Witte et al. (13) described a patient with Angelman syndrome where pain could not be assessed following spinal surgery for scoliosis and a later open reduction and internal fixation for a metacarpal traumatic fracture. In our series we could not retrospectively determine if postoperative pain management was confounded, but no patients were reported to have pain or were administered analgesic medications (Table 2) despite the fact that some procedures had the potential to be painful (i.e., strabismus surgery, adenotonsillectomy). Patil et al. (16) described severe postoperative agitation in a 12 year-old boy following strabismus surgery which was attributed to anxiety, but it could had been from pain. Given this theoretical concern, the presence of parent or guardian familiar with the patient may be helpful in postoperative pain assessments. It should also be noted that sleep disturbances are common with Angelman syndrome, but the effects of anesthesia and surgery on these patients’ sleep patterns have not been described.
Not all patients with Angelman syndrome have profound cognitive limitations. Patient #3 in this series had only mild cognitive impairment, had the ability to verbally communicate, and was seizure-free. In fact, upon reaching adulthood, this patient was able to live semi-independently in a group home setting and was employed. These less severe phenotypical presentations of Angelman syndrome do occur infrequently, and in some cases are due to a mosaic methylation defect of the critical region/locus. Most patients have maternal deletion of 15q11-q13, the Prader-Willi Angelman syndrome critical region, and these patients are typically more severely affected (2, 3, 31). However, paternal uniparental disomy, imprinting defects, or UBE3A gene mutations can also result in Angelman syndrome, and these patients may have a milder clinical presentation (2, 31, 32). We are not certain the exact genetic defect in this patient because her family declined further genetic evaluation.
4.2. GABA-A Receptor Abnormalities
In addition to abnormalities of the UBE3A gene, in some cases the genetic abnormality may also result in a defect of the gene that encodes the β3 subunit of the GABA-A receptor. This may account for some of the neurological features of Angelman syndrome (e.g., seizures, behavior abnormalities) (33). The abnormality may result in decreased central nervous system binding sites for benzodiazepines (34) and flumazenil (35) and unpredictable responses to intravenous anesthetics which exert their affect vis-a-vis GABA-A interactions. One patient in our series (Patient #4) was found to be very sensitive to benzodiazepines. This 16 year, 47 kg girl failed to meet extubation criteria after a 90 minute general anesthetic and 2.5 hour recovery stay and after receiving 20 mg oral midazolam for preprocedural sedation. Her oversedation was promptly reversed with the administration of flumazenil. There have been other reports of prolonged anesthesia recovery following general anesthesia (7, 16, 17, 24). However, of these 4 cases, only Kemper et al. (24) reported the use of midazolam, and other cases used midazolam without incident. Conversely, Bo et al. (8) reported that a 7-year-old girl was administered two 2 mg doses of midazolam preoperatively without effect. It is not clear if these cases of delayed recovery are due to GABA-A abnormalities or because of some other unaccounted condition.
4.3. Seizure Activity
Patients with Angelman syndrome have characteristic electroencephalogram patterns and approximately 80% of patients will have seizure activity (2). Four of our patients had underlying seizure disorders and another one had an abnormal electroencephalogram. Patient #6 had worsening seizure activity during an episode of urosepsis which prompted a diagnostic computer tomography scan under general anesthesia. However, we did not encounter any of our patients or reports in the literature of worsening seizure activity following anesthesia. Anesthesiologists should be aware that many of these patients are on seizure medication and it is probably prudent to not disrupt antiepileptic regimens during the perioperative period.
4.4. Craniofacial Abnormalities
Patients with Angelman syndrome are typically born with normal head circumference but have retarded head growth and often develop microcephaly by the 2nd year of life (2). Other common craniofacial abnormalities include occipital abnormalities, prognathia, wide mouth and wide-spaced teeth. The tongue may protrude and patients can exhibit tongue thrusting and chewing behaviors as well as excessive drooling (2). Because of profound intellectual disability, patients frequently have poor dental hygiene, leading to dental disease that requires interventions (again, under general anesthesia). Some of these issues raise concern that patient may have difficult airways. In our series, Patient #6 was electively intubated by a videolaryngoscope and the supervising anesthesiologist noted that the airway could be difficult to manage with traditional techniques. Bujok et al. reported that a 12 year-old boy had a high arched palate and a disproportionately large and low placed larynx (7). Misumi et al. (28) reported that it took two attempts with direct laryngoscopy to secure the airway in a 20 year-old woman. Lastly, Kemper et al. (24) reported a 3-year-old boy who had the ‘working diagnosis’ of mucopolysaccharidosis but was subsequently determined to have Angelman syndrome. This child had 2 failed attempts at laryngeal mask airway placement which resulted in oropharyngeal bleeding. The airway was ultimately secured with an endotracheal tube.
4.5. Increased Vagal Tone
There are two reports of profound bradycardic episodes in patients undergoing general anesthesia. Bujok et al. (7) reported a case of 12 year-old boy undergoing a dental procedure who developed bradycardia with a heart rate of 40 beats per minute after 65 minutes of an unremarkable general anesthetic. This bradycardia was recalcitrant to 0.6 mg of atropine. Gardner et al. (11) reported a (9) year-old girl who developed bradycardia during the establishment of pneumoperitoneum. It was unresponsive to 0.4 mg atropine and release of the pneumoperitoneum. The slow heart rhythm deteriorated into asystole and the patient required chest compressions and epinephrine to reestablish a heartbeat. This patient required 72 hours of postoperative mechanical ventilation and vasopressor support. During this period frequent episodes of bradydysrhythmias were noted. None of the patients in our series developed dysrhythmias during anesthesia.
There is evidence of increased vagal tone in other settings in patients with Angelman syndrome. Vanagt et al. (10) reported a case of a 12 year-old girl with Angelman syndrome who had recurrent episodes of asystole and syncope in response laughing episodes. Her symptoms were controlled with the administration of atropine. It has been postulated that these outbursts of laughing may results in a Valsalva-like maneuver that may trigger the extreme vagal reaction. Douchin et al. (9) reported 3 children with Angelman syndrome with increased vagal tone manifested by “malaise” or syncopal episodes. Patients had normal electrocardiogram and echocardiograms, but had profound episodes of bradycardia recorded by Holter monitor. Bradycardic episodes were triggered by laughing fits in one child. Two children were treated with diphemanil and the other disopyramide. Unfortunately, one patient suddenly died at age 6 when she had an acute syncopal episode and cardiac arrest, 2 years after discontinuation of diphemanil.
4.6. Muscular Abnormalities
Truncal hypotonia in this patient group frequently presents during infancy. As these children age they may experience increased muscle tone in the limbs (upper > lower) (2). These muscle tone abnormalities raise concern regarding the safety of muscle relaxants (5). In our series Patient #4 was administered atracurium and Patient#6 succinylcholine to facilitate endotracheal intubation, both without incident. Administration of nondepolarizing muscle relaxants have been previously described mostly without incident (8, 11, 12, 16, 17, 20, 28, 30, 36). The one exception was a 40 year-old woman who experienced unexplained upper limb, ‘deltopectoral and truncal rigidity’ when cisatracurium was used to facilitate endotracheal intubation for a dental procedure (17).
4.7. Respiratory Complications
Respiratory abnormalities are not a hallmark feature of Angelman syndrome (2). However, our Patient #2, who had no history of either asthma or reactive airway, experienced a severe bronchospasm during a general anesthetic, but the fact that it resolved with deepening of anesthetic with propofol indicates that provider attributed this event to light anesthesia. Landsman et al. (14) described a 2 year-old girl who underwent an adenotonsillectomy and had increased work of breathing during recovery and overnight developed respiratory failure requiring reintubation and mechanical ventilation. In both these cases it remains unclear whether respiratory problems were related to Angelman syndrome, or represent an inherent risk of respiratory complications for medically-complex pediatric patients undergoing general anesthesia. Maguire et al. (17) report a 40 year-old woman who had erratic breathing patterns postoperatively, but this was in the context of an acute episode of muscle rigidity of the torso during emergence from anesthesia. No cases of aspiration pneumonia were noted despite the tendency of these patients tendency to have excessive drooling (2).
4.8. Conclusion
Angelman syndrome is a rare neurodevelopmental disorder characterized by profound intellectual disability, seizure disorders, GABA-A receptor abnormalities, craniofacial abnormalities, and increased vagal tone. Though the majority of patients in this series and in the published literature tolerated anesthesia well, the anesthesiologist should be aware of the features of Angelman syndrome and alter the anesthetic management accordingly. Despite the uneventful anesthetic course in our patients, small case series cannot provide definitive assurance regarding risks of anesthetic exposure for these patients. However, given the rarity of this disorder, we feel that reports such as ours represents a contribution to gain better understanding of anesthetic management of this debilitating syndrome .