1. Background
In recent years, several studies have been performed to introduce useful drugs in reducing complications of anesthesia during cesarean section (1). Studies have shown that both regional anesthesia and general anesthesia are appropriate and acceptable methods in the cesarean section. Regarding the drawbacks of general anesthesia such as difficult airway or aspiration of gastric contents, regional anesthesia has been accepted as the preferred technique for cesarean section (2). Although various types of regional anesthesia can be used in the cesarean section, spinal anesthesia is the most common method due to its convenience, low drug dosage, rapid onset, and time-efficiency (3). Spinal anesthesia has become a popular method in cesarean as it provides a fast, deep, symmetric and high quality sensory and motor block (3, 4).
One of the most common complications of spinal anesthesia, especially in pregnant women, is hypotension. If effective measures are not taken to prevent it, the incidence of hypotension will be about 80% (5, 6). The main cause of hypotension and bradycardia in spinal anesthesia is the hindered activity of the sympathetic nervous system (7). This can lead to blood pooling in lower limbs and hypotension intensified by the aortocaval pressure of the enlarged uterus. To maintain the maternal cardiac output and arterial pressure, the liberal use of crystalloids and colloids is not sufficient, so the use of vasopressors is required to prevent and treat hypotension (8-10). Recent data do not support the use of ephedrine as the primary therapeutic option to prevent or treat maternal hypotension after spinal anesthesia (11, 12).
Phenylephrine is a selective α1-adrenergic agonist. Its rapid onset and short duration of action have made it an appropriate and effective infusion drug (13). Unlike ephedrine, phenylephrine leads to increased umbilical PH which in turn ensures enough umbilical blood circulation (14). Considering the advantages of phenylephrine, it is currently the first-line choice to prevent spinal hypotension during cesarean (15). Some studies have investigated the phenylephrine usage as bolus or infusion to maintain maternal arterial blood pressure during spinal anesthesia; however, more studies are required to determine the best regimen and dose of phenylephrine with an emphasis on maternal and neonatal outcomes (15, 16).
2. Objectives
This present study compares the efficacy of prophylactic bolus (100 µg) and infusion (50 µg/min) regimens of phenylephrine with the placebo to reduce the maternal hypotension after the induction of spinal anesthesia in cesarean sections.
3. Methods
This is a double-blind, randomized clinical trial was approved by the Research Ethics Committee of the Hamadan University of Medical Sciences and registered in the Iranian Registry of Clinical Trials coded as IRCT201601238768N4. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by The Institution's Human Research Committee. The participants comprised of 120 healthy women undergoing elective cesarean delivery under spinal anesthesia with an ASA class I and singleton term infant referring to Fatemieh Hospital, Hamadan, Iran. Using a block randomization technique with a block size of 6, we categorized the women randomly into 3 groups each with 40 members. Some women were excluded from the study under the following circumstances: laboring women with a history of chronic hypertension, preeclampsia, twin pregnancy, preterm infants, a history of allergy to anesthetics or other medications including phenylephrine, placenta accreta, and percreta, a history of cardiovascular, pulmonary or renal disease, or a history of multiple previous surgeries leading to severe adhesion bands and fibrosis. When the patient arrived at the operating room, a G18 catheter was inserted and 10 mL/kg IV crystalloid (Ringer's lactate) was infused for each patient. Noninvasive BP monitoring, O2 saturation, and ECG monitoring devices were attached to the patients whereby their vital signs were recorded. Spinal anesthesia was induced in the sitting position at either L3-L4 or L4-L5 spaces by injecting 10 milligrams hyperbaric bupivacaine 0.5% and 25 micrograms fentanyl, after which the patient was immediately placed in the supine position. The level of anesthesia was measured by assessing the loss of pinprick discrimination five minutes after the intrathecal injection. The patients’ blood pressure and heart rate were recorded before the induction of spinal anesthesia, immediately after induction and every 1 minute until the end of delivery.
Hypotension was defined as more than a 20% decrease of BP compared to baseline and more than a 20% increase of BP compared to baseline. It was treated with 100 microgram bolus phenylephrine.
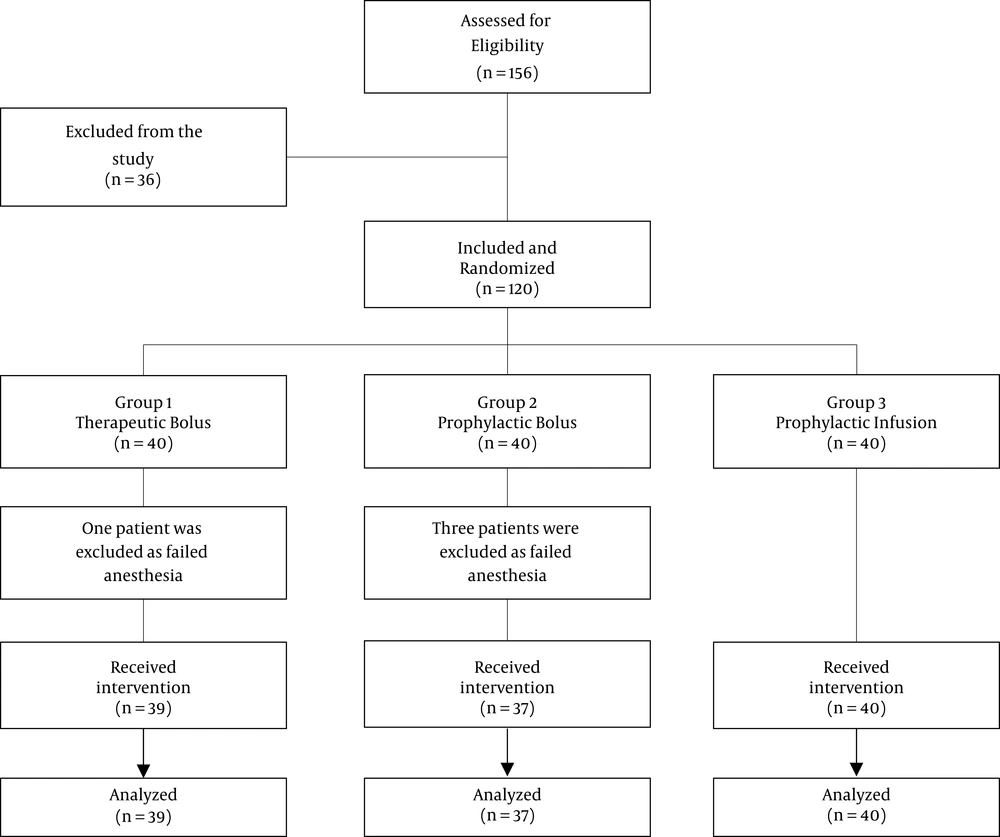
This study consisted of three groups. In the first (control) group, 1 mL bolus of normal saline was injected immediately after the induction of spinal anesthesia. Then the normal saline infusion was started and continued until the delivery of the baby. In the second group, 1 mL bolus of prophylactic phenylephrine (100 μg) was injected immediately after spinal injection and then normal saline infusion was administered until delivery of the baby. In the third group, 1 mL bolus of normal saline was injected immediately after the spinal anesthesia, then prophylactic phenylephrine (50 μg/min) infusion was infused until delivery of the baby. Hypotension observed at any time during surgery in all study groups, would be treated with administration of 100 micro gram phenylephrine. All of the drugs were prepared in similar syringes with the same volume. The patient and the nurse who recorded the vital signs were not aware of the grouping and medications (Figure 1).
Other pieces of information such as the patients’ age, weight, height, gestational age and any incidences of nausea or vomiting, bradycardia (HR < 60), hypotension or hypertension, the number of atropine and bolus phenylephrine requirements and adjuvant phenylephrine dose were recorded in the checklist.
The collected data were analyzed in SPSS V. 22. The normality of data distribution was analyzed using the Kolmogorov-Smirnov test. The ANOVA or Kruskal-Wallis tests were used to compare the quantitative variables between the groups. The qualitative variables between the three groups were compared by the chi-square test.
4. Results
In this study, 120 women were studied in three groups. Most of the subjects were 25 - 35 years old and were in their 37 - 41 gestational weeks. All three groups were similar in terms of maternal age, pregnancy weight, height, gestational age, and block height (P > 0.05) (Table 1). According to the results, HR means showed no significant difference before spinal anesthesia and one to six minutes after spinal anesthesia (P > 0.05). Immediately after the spinal block, the mean of HR was significantly different between groups (Table 1) (P < 0.05). Mean systolic blood pressure (SBP) did not show any significant difference in the three groups (P > 0.05) before and after spinal anesthesia at first, three, four and five minutes after spinal anesthesia. However, the difference between groups were significant in the second and 7th minutes after spinal anesthesia (P < 0.05) (Table 2). The hypotension in the group receiving a prophylactic infusion of phenylephrine, bolus phenylephrine, and control group was 2.5%, 15%, and 35%, respectively. The difference between groups was statistically significant (P = 0.001) (Table 2).
| Variables | Group 1 (Therapeutic Bolus) | Group 2 (Prophylactic Bolus) | Group 3 (Prophylactic Infusion) | P Value |
|---|---|---|---|---|
| Mother’s age (y) | 4.17 ± 34.22 | 1.50 ± 28.10 | 5.82 ± 28.89 | 0.426 |
| Pregnancy weight (kg) | 10.84 ± 72.71 | 12.63 ± 76.05 | 9.47 ± 73.18 | 0.346 |
| Height (cm) | 5.23 ± 162.68 | 36.08 ± 154.15 | 5.74 ± 161.98 | 0.347 |
| Gestational age (wk) | 38.1 ± 3.2 | 38.4 ± 2.2 | 38.6 ± 2.3 | 0.2 |
| Upper sensory level (median, range) | T4 (T2 - T5) | T4 (T2 - T5) | T4 (T2 - T5) | 1 |
| Spinal puncture to delivery (min) | 5.2 ± 8.3 | 5.2 ± 7.8 | 5.2 ± 9.1 | 0.35 |
aValues are expressed as mean ± SD.
| Variables | Group 1 (Therapeutic Bolus) | Group 2 (Prophylactic Bolus) | Group 3 (Prophylactic Infusion) | P Value |
|---|---|---|---|---|
| HR before spinal anesthesia | 100.23 ± 18.01 | 95.23 ± 9.67 | 97.50 ± 14.93 | 0.32 |
| HR after spinal anesthesia | 100.88 ± 19.19 | 94.31 ± 12.506 | 89.50 ± 17.63 | 0.011 |
| HR after two minutes | 91.68 ± 26.33 | 90.10 ± 20.79 | 90.55 ± 22.36 | 0.953 |
| HR after three minutes | 91.23 ± 24.96 | 89.46 ± 20.44 | 87.38 ± 20.36 | 0.674 |
| HR after four minutes | 90.23 ± 19.07 | 91.00 ± 16.91 | 87.60 ± 17.43 | 0.925 |
| HR after five minutes | 90.78 ± 18.27 | 90.51 ± 16.91 | 89.33 ± 17.34 | 0.663 |
| HR after six minutes | 90.93 ± 17.07 | 87.15 ± 15.43 | 89.24 ± 17.08 | 0.689 |
| SBP before spinal anesthesia | 125.08 ± 10.16 | 120.03 ± 8.693 | 122.89 ± 10.22 | 0.075 |
| SBP after spinal anesthesia | 107.50 ± 16.67 | 110.98 ± 15.33 | 113.35 ± 10.22 | 0.191 |
| SBP after two minute | 103.55 ± 15.94 | 109.48 ± 13.96 | 114.03 ± 13.79 | 0.007 |
| SBP after three minutes | 108.48 ± 14.93 | 111.59 ± 13.17 | 114.83 ± 11.92 | 0.112 |
| SBP after four minutes | 112.68 ± 12.88 | 116.88 ± 12.32 | 114.60 ± 10.98 | 0.302 |
| SBP after five minutes | 116.88 ± 11.69 | 117.73 ± 11.40 | 116.93 ± 8.45 | 0.922 |
| SBP after six minutes | 121.23 ± 8.37 | 114.94 ± 14.53 | 118.30 ± 10.01 | 0.094 |
| SBP after seven minutes | 127.08 ± 8.12 | 113.06 ± 8.49 | 117.26 ± 8.19 | 0.001 |
| SBP after 8 minutes | 125.15 ± 7.1 | 118.43 ± 7.3 | 117.49 ± 8.43 | 0.087 |
| SBP after 10 minutes | 109.57 ± 9.5 | 103.37 ± 7.3 | 115.69 ± 8.43 | 0.096 |
| Hypotension: Yes (%) | 14 (35) | 6 (15) | 1 (2.5) | 0.001 |
Abbreviations: HR, heart rate (beats/min), SBP, systolic blood pressure (mmHg).
aValues are expressed as mean ± SD or frequency (%).
Evaluating the secondary outcomes of the patients showed that the groups were not considerably different in terms of nausea and vomiting, bradycardia, the number of atropine requirements, and incidence of hypertension (P > 0.05) (Table 3).
| Variables | Group 1 (Therapeutic Bolus) | Group 2 (Prophylactic Bolus) | Group 3 (Prophylactic Infusion) | P Value |
|---|---|---|---|---|
| Nausea and vomiting | 9 (22.5) | 5 (12) | 4 (10) | 0.2 |
| Bradycardia | 5 (12.5) | 4 (10) | 2 (5) | 0.5 |
| Atropine requirement | 4 (10) | 4 (10) | 2 (5) | 0.65 |
| Phenylephrine bolus requirement | 22 (55) | 15 (37.5) | 4 (10) | 0.001 |
| Hypertension | 4 (10) | 1 (2.5) | 3 (7.5) | 0.39 |
| Adjuvant Phenylephrine dose (µg) | 52.5 ± 50.5 | 50 ± 42.5 | 22 ± 5 | 0.000 |
| Apgar score (first minute) | 8.05 ± 0.876 | 8.33 ± 0.797 | 8.08 ± 0.656 | 0.224 |
| Apgar score (fifth minute) | 9.15 ± 0.736 | 9.20 ± 0.853 | 9.20 ± 0516 | 0.65 |
aValues are expressed as mean ± SD or frequency (%).
The number of phenylephrine bolus requirements was significantly different in all the groups. 10% of patients in the group receiving prophylactic infusion, 37.5% in the prophylactic bolus group, and 55% in the control group needed rescue phenylephrine boluses (P = 0.001). The mean adjuvant phenylephrine dose was significantly different in the three groups, greater in the control group and less in patients receiving prophylactic infusion. The mean ± SD adjuvant phenylephrine dose was 22 ± 5 µg, 50 ± 42.5 µg and 52.5 ± 50 µg in the group receiving prophylactic infusion dose, prophylactic bolus, and control group, respectively (P = 0.000) (Table 3).
5. Discussion
The results of this study showed that prophylactic infusion of phenylephrine (50 µg/min) was more effective in reducing the incidence of hypotension than the prophylactic bolus (100 µg) and placebo. In general, in all-time points, the blood pressure was higher in the phenylephrine infusion group than the other two groups, although the difference of mean SBP between groups was only significant at second and 7th minutes after spinal anesthesia. Comparing the effects of prophylactic variable rate phenylephrine infusion with saline infusion; Siddik-Sayyid et al. concluded that the incidence of hypotension was lower in the phenylephrine infusion group (17). Similarly, Allen et al. found that prophylactic phenylephrine infusion reduced the incidence and severity of hypotension compared to placebo (18). The result of their study on patients undergoing elective femoral fractures surgeries and receiving Hetastarch or Ringer's lactate solutions showed that both solutions had the same effects on the compensation of hypotension, CI, and CO in patients undergoing spinal anesthesia. In a study by Fathi et al., it was shown that both Hetastarch or Ringer's lactate solutions have no effects on hypotension in patients undergoing spinal anesthesia (19).
However, Doherty et al. compared two groups of parturient: a group receiving a fixed-rate (120 µg/min) prophylactic phenylephrine infusion and a group receiving bolus dose (120 µg). They observed no clinical advantage for phenylephrine infusion over bolus one, reporting that the bolus regimen maintained blood pressure closer to baseline, although this did not result in a better clinical outcome (20). In contrast to Doherty et al. study, we observed that in the first minutes after spinal anesthesia, phenylephrine infusion with a dose of 50 µg/min results in more hemodynamic stability and the mean SBP in this group of patients was always greater and closer to baseline. However, the difference of mean SBP between the groups was significant only in the second and seventh minutes after spinal anesthesia. Compared to the other groups; the mean SBP in the phenylephrine infusion group was significantly higher in the second minute and significantly lower in the seventh minute (Table 3).Significant lower SBP in the seventh minute in the phenylephrine infusion group can be justified by receiving additional rescue phenylephrine doses in other groups.
Although HR was always lower in the prophylactic phenylephrine infusion group than the other two groups in our study, there was no significant difference in the mean HR between the three groups. Faiz et al. examined the effects of intrathecal injection of magnesium sulfate (MgSO4) to bupivacaine on perioperative shivering in patients undergoing elective cesarean section. No significant difference was observed in the mean of the heart rate between the groups at various time points after blocking (21).
Our finding corresponds with the study by Siddik-Sayyid et al. in which they observed no significant difference in HR between the prophylactic phenylephrine infusion and the therapeutic phenylephrine bolus group (17). Faiz et al., assessed the anesthetic effects of adding intrathecal neostigmine or magnesium sulphate to bupivacaine in patients under lower extremities surgeries. As a result it was indicated that the homodynamic status was not significantly different across the study (22).
The three groups in our study had no significant differences in perioperative nausea and vomiting. In a study by George et al., compared the incidence of intraoperative nausea and vomiting in obese patients who received a prophylactic phenylephrine infusion versus those who received phenylephrine boluses undergoing cesarean delivery. Their results revealed that there was no difference in the incidence of intraoperative vomiting between the two groups (23).
This result was significantly different from the findings of Siddik-Sayyid et al., as nausea and vomiting in their study were significantly lower in the prophylactic infusion group than the bolus group (17). Similarly, in another study by Ngan Kee et al. on the different prophylactic phenylephrine regimens, patients with 100 µg/min infusion dose experienced less nausea and vomiting than the smaller doses of 80 and 90 µg/min phenylephrine infusion (24). Similar to Siddik-Sayyid et al. (17) and Doherty et al. (20) studies, atropine requirement and bradycardia were not significantly different in the patients who received prophylactic phenylephrine infusion in our investigation. We found that patients in the control group and prophylactic phenylephrine bolus need more rescue phenyllephrine doses, and the mean adjuvant phenylephrine doses were greater in these two groups to keep SBP near baseline compared to the infusion group. Although phenylephrine infusion has resulted in greater hypertension in other studies (17, 18, 20), the incidence of hypertension did not differ significantly between phenylephrine infusion and other groups in our study which was consistent with the studies of Heesen et al. (16) and Ngan Kee et al. (24). Comparing different phenylephrine infusion regimens, infusion of 100 µg/min phenylephrine leads to fewer hypotensive episodes than 80 and 90 µg/min doses (24). Although we used smaller phenylephrine infusion dose (50 µg/min) in our study; patients in infusion group saw significantly lower incidence of hypotension compared to bolus and control groups. The higher mean SBP in second minute in control group could be explained by more rescue phenylephrine injection in this group of patients. Apgar score in the first and fifth minutes also did not differ in the three groups in our study which was consistent with other studies (17, 24).
5.1. Limitations
The limitations of the present study included the complete blinding and preparation of placebo, drug syringe pumps, and repeated blood pressure measurements.
5.2. Conclusions
The results of the present research suggested that prophylactic phenylephrine infusion (50 µg/min) reduced the incidence of hypotension more effectively than the prophylactic bolus and therapeutic bolus groups in women undergoing spinal anesthesia for cesarean section, and provided a better hemodynamic stability for the mothers. Nonetheless, more studies are needed to consider other aspects of mother and neonatal well-being in this area.