1. Background
In patients admitted to the ICU, Enteral Nutrition (EN) is the first choice for feeding support, however, it is often complicated by gastrointestinal side effects, such as diarrhea (1, 2). It has been determined that diarrhea occurs in approximately 15% to 18% of critically ill patients, who receive enteral nutrition, compared to only 6% of patients, who don’t receive enteral nutrition (3). The precise mechanism is unknown, yet alteration of intestinal transit or the intestinal microflora has been proposed. Studies have shown that standard formulas, which are without fiber and prebiotics, reduce total colonic bacteria and increase numbers of aerobes (4, 5). They also decrease the numbers of butyrate-producing Faecalibacterium prausnitzii, resulting in the reduction of total small chain fatty acids and butyrate and higher fecal pH (4, 6). Prebiotics are typically non-digestible fiber compounds that induce the growth or activity of bacteria in the colon and it is said that they are useful for health (7). There is no study, which has specifically evaluated the effect of a prebiotic, which prevents diarrhea during enteral nutrition; yet some studies have investigated the effect of a fiber/prebiotic formula with diverse effects (8-11). Honey, as a prebiotic, contains oligosarccharides or bifidogenic factor, besides a wide range of other valuable nutrients (12). It is also a good source of carbohydrate and has antibacterial property (13). It has been shown that honey reduces prostaglandin levels and increases nitric oxide levels (14). The antimicrobial effect of honey on bacterial diarrhea has been demonstrated in a few studies (15, 16). No study has indicated the preventive effects of honey on diarrhea and alterations in fecal microbiota in patients hospitalized at the Intensive Care Unit (ICU). In view of this, the current study selected ICU patients, who received enteral nutrition to evaluate these effects.
2. Materials and Methods
The researchers surveyed 32 patients above 18 years old, hospitalized at a tertiary care university hospital from September 2012 to August 2013. Initially, the protocol was recorded and the code of ethics was obtained. Also, the researchers obtained informed consents from all patients or their legal guardians. Exclusion criteria were age of less than 18 years, a history of gastrointestinal disease, digestive tract surgery, diabetes, intestinal obstruction, paralytic ileus, intestinal ischemia, septic patients, and hyperthyroidism. No prebiotic or laxatives (such as lactulose and lactitol) were permitted. All patients were to receive more than 75% of their total energy within 48 hours and their antibiotic regimens were the same. subjects were in the study for 7 days or more.
The researchers recorded the patient’s past and present medical history, physical examination, Acute Physiology and Chronic Health Evaluation II (APACHE) score, and primary diagnosis. After enrolment, subjects were randomized to 2 groups. All participants were fed via a nasogastric tube with a high-protein standard kitchen formula prepared at the hospital in an intermittent fashion. The distribution of macronutrients was 20% for protein, 30% for lipid, and 50% for carbohydrate. In the study group, 10% of the carbohydrate content was from natural honey. The subjects, researchers, and all clinical personnel remained blinded to the randomization.
2.1. Assessment of Clinical Outcomes
The following data were collected from each patient: occurrence of diarrhea, occurrence of sepsis, multiple organ failure syndrome, and number of ICU hospitalization days. Diarrhea was defined as liquid stools for more than 3 times during 2 or more consecutive days. American College of Chest Physicians (ACCP) and Society of Critical Care Medicine (SCCM) consensus conference defined sepsis (17). Occurrence of Multiple Organ Failure Syndrome (MOFS) was monitored during hospitalization. Sequential Organ Failure Assessment (SOFA) score was used to determine the extent of a person's organ function. Each patient was evaluated for cardiovascular failure (systolic blood pressure of ≤ 90 mm Hg or required vasopressor support), central nervous system failure (Glasgow coma score of ≤ 12), coagulation failure (platelet count of ≤ 80 × 109/L), hepatic failure (bilirubin of ≥ 2 mg/dL), and renal failure (serum creatinine of ≥ 2 mg/ dL or 25% rise from the baseline). Patients were also followed for mortality during hospitalization.
2.2. Assessment of Nutritional Variables
During the study the daily energy intake from tube feeding was recorded for each patient. Serum albumin level was measured on day 0 and 7 for each patient.
2.3. Laboratory Data
Stool samples were taken on study days 0 and 7 for the measurement of Lactobacillus and Bifidobacteria. About 500 mg of each stool sample was taken from the center of the stool, frozen immediately, and stored at -20°C. Bacterial DNA from fecal samples was extracted using the QIAamp DNA Stool Kit (Qiagen). The DNA extract was analyzed spectrophotometrically to determine purity and concentration. The primer sequences used for quantitative real-time PCR were obtained from previous studies (18-20) (Table 1).
| Target Organism, Primer Set | Primer Sequence | Product Size, bp | Annealing Temperature | Reference |
|---|---|---|---|---|
| All bacteria | ||||
| Forward | 5-TCC TAC GGG AGG CAG CAG T-3 | 466 | 59 | (18) |
| Reverse | 5-GGA CTA CCA GGG TAT CTA ATC CTG TT-3 | 58 | ||
| All bifidobacteria | ||||
| Forward | 5-GGG TGG TAA TGC CGG ATG-3 | 457 | 59 | (19) |
| Reverse | 5-TAA GCC ATG GAC TTT CAC ACC-3 | |||
| Lactobacillus | ||||
| Forward | 5-AGCAGTAGGGAATCTTCCA-3 | 341 | 58 | (20) |
| Reverse | 5-CACCGCTACACATGGAG-3 |
2.4. Real-time Polymerase Chain Reaction
Real time PCR was performed using Maxima SYBR Green/Flouresceinq PCR master mix (Thermo Scientific) in a total reaction volume of 25 mL consisting of 500 mg extracted DNA, 1 µM of each primer, and 13.3 mL of Maxima SYBR Green/Flouresceinq PCR master mix. Amplification was performed in a Mini Opticon (BioRad) thermocycler with the following conditions: (1) an initial denaturation step of 3 minutes at 95°C; (2) 37 cycles, each consisting of 30 seconds at 95°C and 60 seconds at 58°C (all bacteria and lactobacillus) or 60°C (bifidobacteria). Fluorescence was measured at the end of the elongation step of each cycle. A melt curve analysis was performed between 55°C and 95°C with an increment of 1°C per 10 seconds. No Template Control (NTC) consisting of H2O for target and reference genes were included in each run. The PCR products were also checked on 12% polyacrylamide gel to demonstrate that the PCR yielded a unique band. For data analysis, delta Ct (ΔCt) was calculated for each target gene (ΔCt = target gene Ct – all bacteria Ct). Since patients had 2 fecal samples (before and after enteral nutrition), in each patient ΔCt was calculated for samples of pre and post nutrition. Next, ΔΔCt was also calculated (ΔΔCt = ΔCtpre nutrition -ΔCtpost nutrition). Statistical analysis was performed between ΔΔCts of placebo and intervention groups. Additionally, ΔCts of pre and post nutrition in each group were compared.
2.5. Statistical Analysis
All statistical analyses were performed with the SPSS software, version 18. Demographic data and baseline values and outcome measures were compared with student’s t test or Mann-Whitney for all continuous variables. The research compared certain data between the groups using the Wilcoxon test. Paired t tests were used for comparisons of variables before and after the intervention. Findings were reported as mean ± Standard Deviation (SD). A P value of ≤ 0.05 was considered statistically significant.
3. Results
Overall, 37 patients participated in this study. Two patients in the study group and 3 patients in the control group were excluded from the study due to discharge before day 7. Therefore, a total of 16 patients in the honey group and 16 patients in the control group completed the survey.
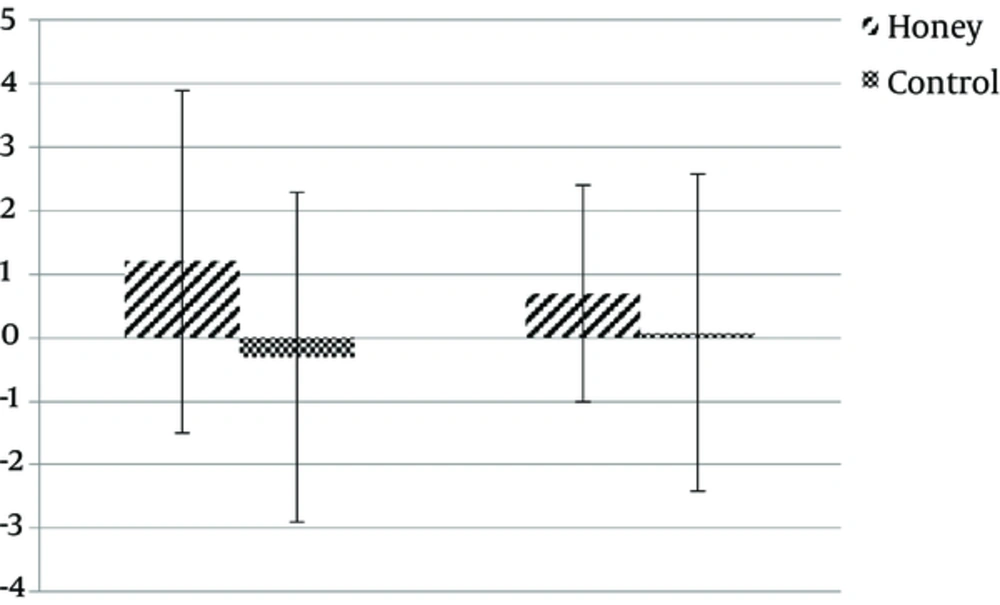
The mean age of subjects was 36.5 years (range: 23 to 49, SD: 8.7). Baseline characteristics of subjects are shown in Table 2. There was no difference in the amount of feeding tolerated during the study (92% versus 93% P = 0.61). Also, there was no difference in the amount of serum albumin level between the 2 groups on days 0 and 7 (P > 0.5). Patients developing diarrhea were 12.5% in total, 6.3% in the honey group and 18.8% in the control group, with a trend towards a decrease in diarrhea in the honey group (P = 0.09). Length of ICU-stay days was significantly lower in the honey group compared with the control group (5.5 ± 1.2 versus 7.3 ± 1.3, P = 0.001). There was a considerable difference in SOFA score for developing organ failure between the honey and control groups (5.3 ± 1.07 versus 6 ± 0.6, P = 0.04). Sepsis was not identified in the 2 groups. The overall in-ICU mortality was 21.8% with 3 deaths in the study group and 4 deaths in the control group, with no significant difference (P = 0.1). Baseline frequency of bifidobacterium and lactobacillus DNA were not significantly different between the honey and control group. Patients in the honey group showed an insignificant increase in the frequency of bifidobacterium DNA by study day 7 in comparison with the control group (Table 2). The frequency of lactobacillus did not differ significantly between the 2 groups on day 7 (P = 0.6). Comparisons of before and after the intervention changes in each group showed that fecal DNA abundance of bifidobacteria and lactobacillus in patients that received honey or placebo in their enteral nutrition did not have statistically significant changes, although, it tended to increase in the honey group (1.2 Ct increase in abundance of bifidobacteria DNA and 0.7 Ct increase in lactobacillus DNA), while those in the placebo group showed no changes or a slight decrease. In addition, fecal DNA of bifidobacteria or lactobacillus species did not differ between the 2 groups (Figure 1).
aAcute Physiology and Chronic Health Evaluation II Score.
bNo significant.
cChronic obstructive pulmonary disease.
Delta Ct (∆∆Ct) refers to bacterial DNA changes in each group after intervention and was calculated as follows: ∆∆Ct = (∆Ctpre nutrition ∆Ct post nutrition), in which ∆Ct is relative expression of each target gene (Bifidobacteria or Lactobacilli), normalized according to the total amount of bacteria (∆Ct = target gene Ct all bacteria Ct).
4. Discussion
The study showed that enteral nutrition with honey could decrease the duration of stay at the ICU. Also, there was a decrease in development of Multiple Organ Dysfunction Syndrome (MODS) and a trend towards a decrease in diarrhea in the honey group. In critically ill patients, gut microflora was altered due to several factors, such as changes in circulating stress hormones, gut ischemia, immunosuppression, the use of antibiotics and other drugs, possible bacterial translocation, and the lack of nutrients (21). Honey, as a prebiotic, stimulates the growth of endogenous useful microorganisms, such as bifidobacteria and lactobacilli, which protects the intestinal tract from the proliferation of pathogenic bacteria and diarrhea. These bacteria inhibit growth of pathogenic bacteria by producing specific antimicrobial agents and volatile fatty acids that acidify the bowel (22, 23). A few studies have shown that honey could also shorten the duration of developing bacterial diarrhea (16, 24).
In this study, there was a trend towards an increase in the frequency of bifidobacterium DNA by study day 7. Sanz et al. showed that fructooligosaccharides of honey increases the populations of bifidobacteria and lactobacilli with its potential prebiotic activity (prebiotic index values between 3.38 and 4.24) in vitro (25). In the current study there was no difference in the frequency of lactobacillus DNA before and after the trial with honey and also between the 2 groups. This may be due to the fact that although Prebiotics stimulate the growth of bifidobacteria and lactobacilli, yet because populations of bifidobacteria are more than lactobacilli in the colon, changes in bifidobacteria are more apparent compared with lactobacilli (24). Other studies have shown that prebiotics specifically increase bifidobacterial populations in fecal samples of humans, and populations of lactobacilli are increased significantly in the fecal microbiota of rodents, such as rats and mouse (26). A reason for the insignificant increase in the populations of bifidobacteria and lactobacilli in the current study may be due to the consumption of broad spectrum antibiotics in the patients. Therefore, the effect of honey on reducing the frequency of diarrhea may be due to other antimicrobial components in honey. Hydrogen peroxide, low pH, methylglyoxal, and antimicrobial peptide bee defensin-1 are recognized as important antibacterial compounds in honey (14, 27). In the current study it was found that the duration of stay at the ICU was significantly lower in the study group versus the control group. Also, the development of MODS was lower in the study group. Multiple hypotheses have been proposed to explain the development of MODS. It appears that bacterial translocation due to disruption of the gut barrier function could be a critical component to the development of SIRS, sepsis, MODS, and duration of stay at the ICU. Thus, the introduction of foods that improve gut-barrier function might prevent the pathogen bacteria translocation process. Prebiotics enhance immune function by activating leukocytes in the gut-associated lymphoid tissue (GALT) system (28-30), increasing number of cells in Peyer’s patches (30), enhancing production of bacteriocins (31) and IgA levels in the small intestine and caecum (32), and improving gut-barrier function (30, 31). Excessive production of pro-inflammatory cytokines and other mediators of inflammation is another hypothesis for developing SIRS and MODS (33). Honey has anti-inflammatory properties. This property is mainly due to its flavonoid compounds (34). On the other hand, oxygen-derived free radicals play an important role in the development of complications in patients admitted to the ICU. Honey also has antioxidant compounds. Phenolic compounds and flavonoids of honey are responsible for its antioxidant capacity (34). Thus, they may be responsible for shorter duration of hospitalization in the study group. There is are not studies, which have specifically evaluated effect of a prebiotic which prevents, diarrhea during enteral nutrition. However, some studies have shown the beneficial effect of a synbiotic/prebiotic formula in ICU patients (35-41); it seems that higher doses of prebiotic may show more significant effects.
4.1. Conclusion
The current study attempted to indicate a clinically significant effect on outcome measures, yet the decrease in length of stay at the ICU and SOFA score and the trend towards a decrease in incidence of diarrhea observed in the treatment group deserves further attention in larger trials. Thus, more extensive studies besides a greater dose of honey are needed to assay these benefits, and if confirmed, honey could be a carbohydrate source of enteral formulas besides its other beneficial compounds.