1. Background
Shivering in surgery is a common and unpleasant complication during the operation, occurring in 6% to 65% of patients, including involuntary movements of one or more muscle groups (1). However, various causes such as pain, decreased sympathetic activity, uncontrolled spinal reflux, release of pyrogen, cytokines following surgery, or adrenal suppression, etc. have been suggested as causative factors of shivering, however, thermo-regulation disorders caused by hypothermia (0.5 - 1°C decrease in central heating) has been accepted as the most common factor (2).
Shivering interferes with pulse oximetry monitoring, measurement of blood pressure and ECG, and increases oxygen consumption up to 5%, which in turn increases the need for cardiac output and respiratory ventilation, which can be found in older patients or in patients with a disease of cardio respiratory tract that causes respiratory heart failure (3). Shivering also causes lactic acidosis, increases carbon dioxide production and muscle fatigue, and increases the internal pressure of the eye (IOP) and intracranial pressure (ICP). In patients undergoing surgery, shivering increases the pain in the site (2, 3). It is important to note that the incidence of shivering in regional anesthesia is not less than general anesthesia and patients' hypothermia continues until the blocking effects disappear below the block level. In regional anesthesia, not only is posting sensory signals from the lower limbs to the impaired centers of nervous system is disturbed, but due to the paralysis of the muscles of the lower limbs, the actual production of heat decreases. As a result, in regional anesthesia, the patient's re-warm-up period is approximately twice in comparison to common anesthesia (3, 4).
Today, various pharmaceutical and non-pharmaceutical solutions have been devised and used to prevent and treat hypothermia as well as shivering. Non-pharmacological methods include the prevention of hypothermia using warm-up blankets and inhaling hot and humid oxygen, keeping the patient warm before and during operation, and preventing the operation room from cooling down. As noted, one of the non-pharmacological methods is to warm the skin, due to the fact that the body's heat regulation system tolerates the central body hypothermia when the skin is warmed; however, this method is effective when the temperature of the central part of the body is greater than 35 °C (5).
Pharmaceutical methods mainly affect the decrease of the shivering temperature threshold. Various medications such as meperidine, clonidine, ketanserin, magnesium sulfate, physostigmine, and etc. have been used to treat shivering. Each of these drugs, although effective in decreasing shivering with various mechanisms, has been shown to have unwanted side effects, such as reducing consciousness and weakening of the respiratory center, as well as causing nausea, vomiting and itching, which is unpleasant for the patient, surgeon and an anesthetist during surgery and will sometimes be life-threatening to the patient (6). Therefore, considering the probable occurrence of these complications, alternative drugs have always been considered for the prevention and treatment of postoperative shivering. In recent studies, the effect of dexmedetomidine on shivering has been continued to study and studies in this area (7-9). Dexmedetomidine, an alpha-2 agonist, is a relatively new sedative drug approved by the FDA and approved for use up to a maximum of 24 hours at a maximum dose of 0.7 μg per kg body weight per minute. Alpha-2 agonists have been shown to have analgesic properties. In addition, dexmedetomidine is used in humans for safe and effective central and peripheral blocks (10) and is widely used in intensive care units (11, 12). Considering the tolerance of addicted patients to narcotic drugs and taking into account the fact that these patients need more analgesic analysts than non-compliant patients during and after surgery (13). In addition, according to a study conducted so far, no study has been conducted to evaluate the anti-shivering effect of this drug in patients with substance abuse.
2. Objectives
The purpose of this study was to investigate the effect of dexmedetomidine prescription on the incidence of shivering during surgery in a spinal anesthetic technique under elective orthopedic surgery of the lower limb in addicted patients.
3. Methods
The study was a double blind randomized clinical trial. The population under study was a candidate addicted patient for lower limb orthopedic surgeries referring to the operating room of Taleghani Hospital affiliated to Shahid Beheshti University of Medical Sciences in 2015. According to similar studies and bass on α = 0.5, β = 0.8, (d = µ1 - µ2 = 1.2), according to the following formula, the sample size was estimated at around 21 people. However, in order to achieve better results, 30 people were considered for each group.

The sampling method was simple non-random. All eligible individuals were selected accordingly, in order to complete the sample. They were randomly divided into 2 groups of 30 patients with dexmedetomidine and normal saline (control). The following entrance criteria were included in the present study: abuse of substances during the past 6 weeks (14), the history of opioid addiction (an inhaler used in opium and 2 peas or 390 mg per day), ages 18 to 55 years, ASA I-II, lower limb orthopedic surgery under spinal anesthesia, lack of underlying disease, such as ischemic heart diseases, diabetes mellitus, and hypertension, body temperature within normal limits, absence of sweating, seizures, tremor or Parkinson's, lack of taking NSAIDs 24 hours before surgery, absence of contraindications of dexmedetomidine prescription and absence of side effects after taking dexmedetomidine, lack of or wound infection, there is no known allergies, the absence of pregnancy and lactation, and duration of surgery up to 3 hours. Patient satisfaction and exclusion criteria include: complications requiring intervention within 24 hours after surgery, patients receiving blood during surgery and anesthesia, and reducing the patient's central body temperature to 32° during surgery. The ethical criteria in this study was approved with the approval of the ethics committee of Shahid Beheshti University of Medical Sciences with the ethics code of IR.SBMU.SM.REC.1394.95.
After the diagnosis of the patient as an appropriate case, the method of implementation and the benefits and possible complications of participating in the project was described, and the patient was given written consent. After obtaining the necessary criteria, they were randomly divided into 2 groups: dexmedetomidine and normal saline (n = 30) and were matched for age and sex. Patients were visited by an anesthetist after entering the operating room, and then, their ASA class was determined. First, a peripheral vein was taken from the patient and 10 cc/kg of lactate ringer serum (warmed to 37°C by Warmer) was given to patients for about half an hour before spinal anesthesia. All patients were under spinal anesthesia with spinal cord number 25 in L3 - L4 or L4 - L5 space with 20-mg bupivacaine hyperbaric, immediately after spinal anesthesia with pupiracaine. Then, dexmedetomidine (0.5 μg/kg/h) and normal saline infusion was given to patients immediately after induction of anesthesia and before surgery by an anesthetist (7, 8). Medicine, a normal saline solution, or placebo was sucked by another doctor in order to not be informed and was delivered to an anesthetist.
The presence of shivering or absence of shivering during the operation (5, 10, 15, 30, 60, 90, 120, 150, 180 minutes) was recorded in terms of the length of operation time, individually. Shivering intensity was also recorded according to Table 1 in each group by an anesthetic technician who did not know the type of prescribed drug. The presence or absence of possible side effects was monitored including nausea, vomiting, water fevers, dry mouth, and itching. In addition, changes in vital signs (including blood pressure, pulse rate, respiratory rate, body temperature) and arterial oxygen saturation in these intervals were evaluated and recorded in the checklist of the researcher. Measurement of the central temperature of the body (tympanic curvature) before and after the injection was performed during and after surgery.
| Class | Status |
|---|---|
| 0 | Without shivering |
| I | Exfoliation of hair or environmental Vasoconstriction (no visible shivering) |
| II | Existence of muscle activity only in a muscle group |
| III | Muscular activity in more than one muscle group that does not occur in general |
| IV | it is included the shivering status of Total Body |
Classification of Shivering Intensity in Patients (11)
The data was collected from patients in a checklist that was recorded by taking the patient's records, clinical examinations, frequency and severity of shivering, and possible side effects of the drug during surgery. Thermometry of patients was performed through tympanic screening. The percentage of arterial oxygen saturation was recorded with a pulse oximeter attached to the finger of the patients. Measurement of vital signs was performed in a sleep condition and was performed by a physician and a tool (cardiac monitor).
In analyzing the data, the mean, standard deviation, frequency, table, and charts was used to categorize and summarize the collected data. In the study of statistical pre-requisites, the Kolmogorov-Smirnov test was used to verify the distribution of data considering the number of observations in each distribution. Regarding the existence of statistical hypotheses, independent t-test and duplicate variance analysis were used through using the statistical package of version 22 (P ≤ 0.05).
4. Results
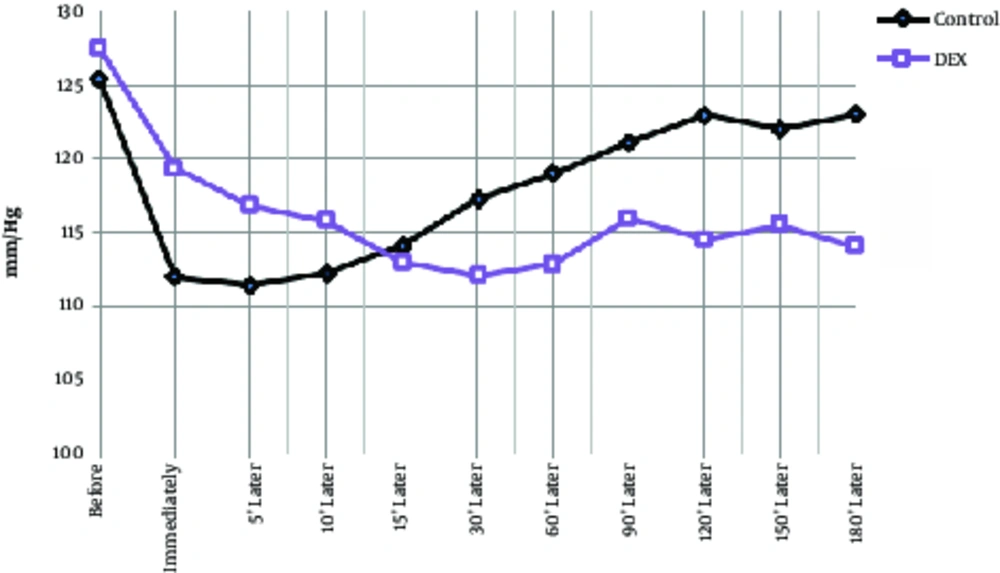
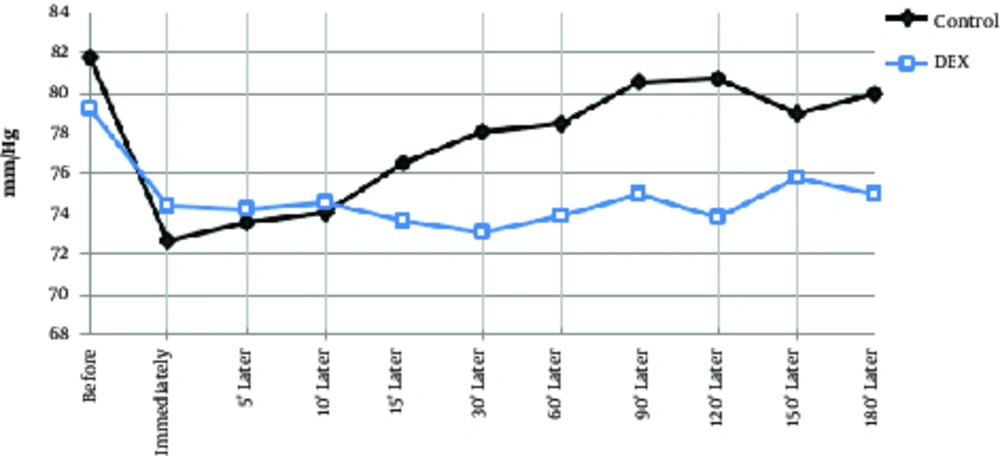
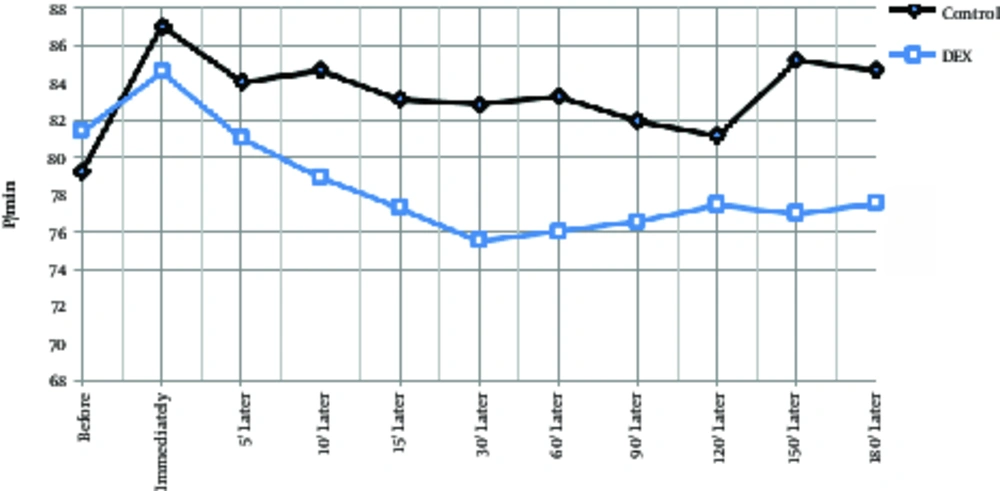
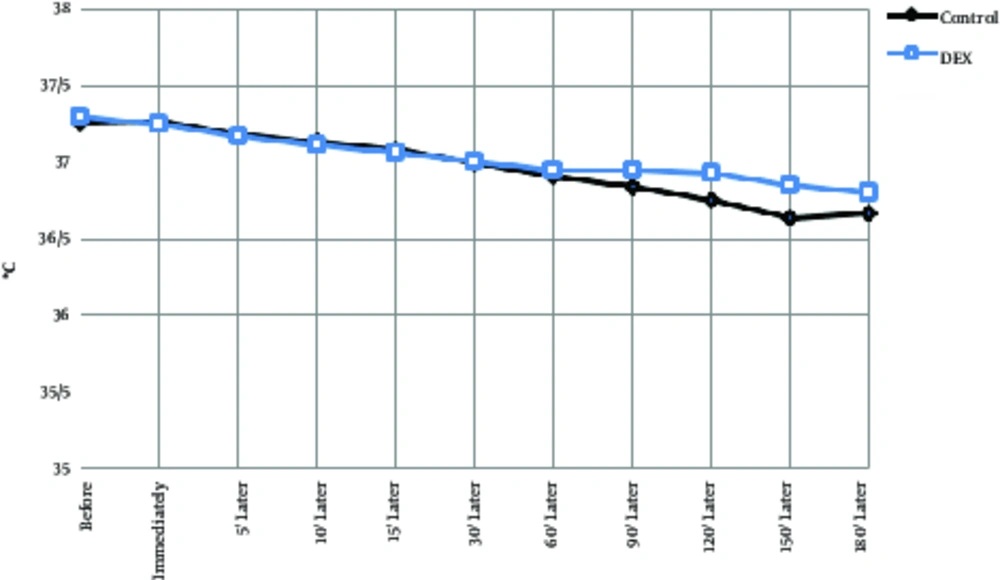
The mean age of the dexmedetomidine group was 32.1 ± 6.8 years and the control group was 34.9 ± 6.3 years, which had no significant difference between them (P > 0.05). A total of 55 patients were male and the rest were female, however, there was no significant difference between the 2 groups in terms of sex due to matching (P > 0.05). During the operation, systolic blood pressure was significantly lower in the dexmedetomidine group at 30 minutes to 150 minutes after injection (Table 2, P < 0.01), and at the other times, there was no significant difference between the 2 groups. In the dexmedetomidine group, the systolic blood pressure significantly decreased immediately after injection and during the operation in comparison to the pre-injection group and in comparison with the control group. In the control group, systolic blood pressure was decreased at all times than before the operation, however, this decrease was significant except for 15, 120, 150, and 180 minutes (Table 2, Figure 1, P < 0.05). Diastolic blood pressure decrease in the control group had a compensatory and increasing condition after 5 minutes and in the dexmedetomidine group, after 60 minutes of starting surgery. In intra-group comparisons in both groups, diastolic pressure drop significantly decreased to 90 minutes after surgery. In general, at other times in each group, diastolic pressure changes were not significant compared to preoperative. In the between group comparison, at 30 minutes to 150 minutes, diastolic pressure drop was significantly lower in the DEX group than in the control group and did not differ significantly at other times (Table 2, Figure 2). Changes in heart rate during the operation in each group ranged from 75 - 88 beats per minute. In both groups, the heart rate was decreased immediately after the injection to 60 minutes after the operation and then, was increased. In the between group comparison, the mean heart rate was significantly lower in the dexmedetomidine group at 30 minutes and 60 minutes compared to the control group and at other times, its lower values were not significantly different with the control group. In intra-group comparison, a significant decrease in pulse rate was observed in preoperative surgery in both groups except for 180 minutes after surgery (Table 2, Figure 3).
| Variable/Group | Measurement Steps | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before the Operation | Immediately After the Operation | 5′ Later | 10′ Later | 15′ Later | 30′ Later | 60′ Later | 90′ Later | 120′ Later | 150′ Later | 180′ Later | |
| Systolic blood pressure | |||||||||||
| Control | 125.4 ± 10.8 | 112 ± 15 | 111.4 ± 11 | 112.2 ± 11.8 | 114.1 ± 9.83 | 117.3 ± 8.9 | 118.9 ± 8.9 | 121 ± 7.4 | 122.9 ± 7.3 | 123.5 ± 10 | 131 ± 1.7 |
| Dexmedetomidine | 127.4 ± 13.2 | 119.3 ± 14 | 116.8 ± 13 | 115.8 ± 10.6 | 112.9 ± 8.9 | 112.1 ± 7.8a | 112.8 ± 4.7a | 116 ± 8.4a | 114.5 ± 9a | 115.4 ± 8.5a | 121.4 ± 7.7 |
| Diastolic blood pressure | |||||||||||
| Control | 81.7 ± 8.3 | 72.7 ± 5.3 | 73.5 ± 7.1 | 74.1 ± 5.2 | 76.5 ± 5.7 | 78.1 ± 7.4 | 78.5 ± 5.5 | 80.5 ± 4.2 | 80.7 ± 3.9 | 81.6 ± 4.5 | 81.3 ± 3.2 |
| Dexmedetomidine | 79.2 ± 9.4 | 74.4 ± 7.1 | 74.2 ± 7.5 | 74.5 ± 6.5 | 73.6 ± 6.4 | 73.1 ± 6.3a | 73.9 ± 6.2a | 75 ± 5.1a | 73.8 ± 5.1a | 75.8 ± 6.2a | 79.5 ± 9.5 |
| Heart rate changes | |||||||||||
| Control | 79.2 ± 13.1 | 87 ± 13.8 | 84 ± 11.6 | 84.6 ± 12.2 | 83.1 ± 12 | 82.8 ± 11.9 | 83.2 ± 12.4 | 81.9 ± 12.3 | 81.1 ± 11.2 | 85.1 ± 9.7 | 84.6 ± 11 |
| Dexmedetomidine | 81.3 ± 10.6 | 84.5 ± 9.8 | 81 ± 12 | 78.9 ± 11.3 | 77.3 ± 11.1 | 75.5 ± 10.1 | 76 ± 10.5 | 76.5 ± 9.2 | 77.4 ± 8.9 | 78.7 ± 8 | 83.1 ± 5.3 |
| Number of breaths | |||||||||||
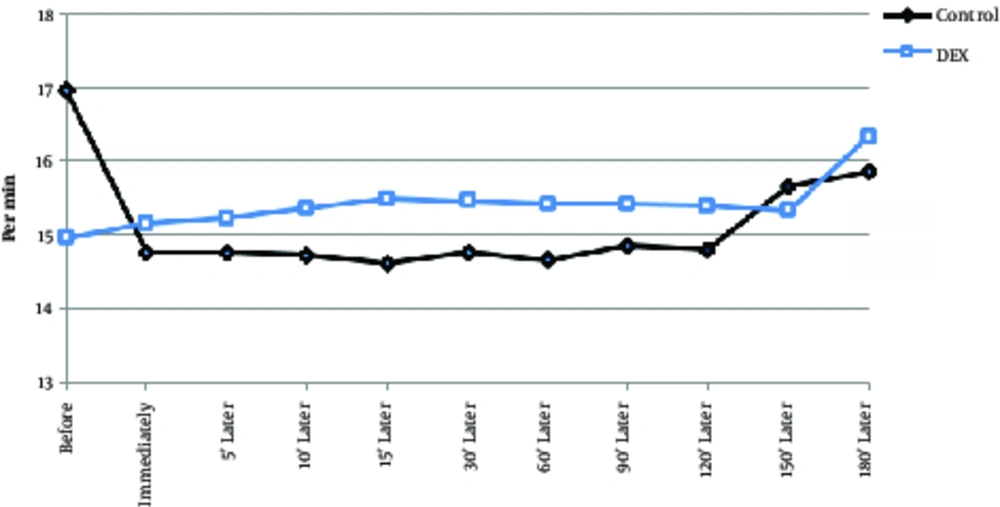
| Control | 15 ± 1.9 | 15.1 ± 1.8 | 15.2 ± 1.4 | 15.3 ± 1.4 | 15.5 ± 1.4 | 15.4 ± 1.3 | 15.4 ± 1.5 | 15.4 ± 1.3 | 15.4 ± 1.1 | 15.3 ± 1.4 | 16.3 ± 1.5 |
| Dexmedetomidine | 17 ± 12.4 | 14.7 ± 2.2 | 14.7 ± 2.2 | 14.7 ± 1.8b | 14.6 ± 1.9 | 14.7 ± 2.06 | 14.6 ± 1.8b | 14.8 ± 1.8 | 14.8 ± 1.7 | 15.6 ± 1.3 | 15.8 ± 1.8 |
| Body temperature changes | |||||||||||
| Control | 37.3 ± 0.2 | 37.2 ± 0.2 | 37.1 ± 0.2 | 37.1 ± 0.2 | 37 ± 0.2 | 36.9 ± 0.3 | 36.9 ± 0.2 | 36.8 ± 0.2 | 36.7 ± 0.3 | 36.6 ± 0.2 | 36.6 ± 0.2 |
| Dexmedetomidine | 37.2 ± 0.3 | 37.25 ± 0.3 | 37.1 ± 0.4 | 37.1 ± 0.4b | 37 ± 0.3 | 37 ± 0.3 | 36.9 ± 0.2b | 36.9 ± 0.2 | 37 ± 0.2 | 36.8 ± 0.2 | 36.8 ± 0.2 |
| Arterial oxygen saturation | |||||||||||
| Control | 98.6 ± 1.2 | 98.9 ± 1 | 98.8 ± 0.9 | 98.8 ± 0.9 | 98.8 ± 0.9 | 98.7 ± 0.9 | 98.7 ± 1 | 98.6 ± 0.9 | 98.7 ± 0.9 | 98.3 ± 0.9 | 97.3 ± 0.5 |
| Dexmedetomidine | 98.4 ± 1.4 | 98.5 ± 1.3 | 98.6 ± 1.3 | 98.6 ± 1.2 | 98.5 ± 1.3 | 98.5 ± 1.2 | 98.6 ± 1.1 | 98.7 ± 1.1 | 98.7 ± 1.1 | 98.6 ± 1.1 | 97.5 ± 1.1 |
Descriptive Statistics and Independent T-Test Between Two Groups
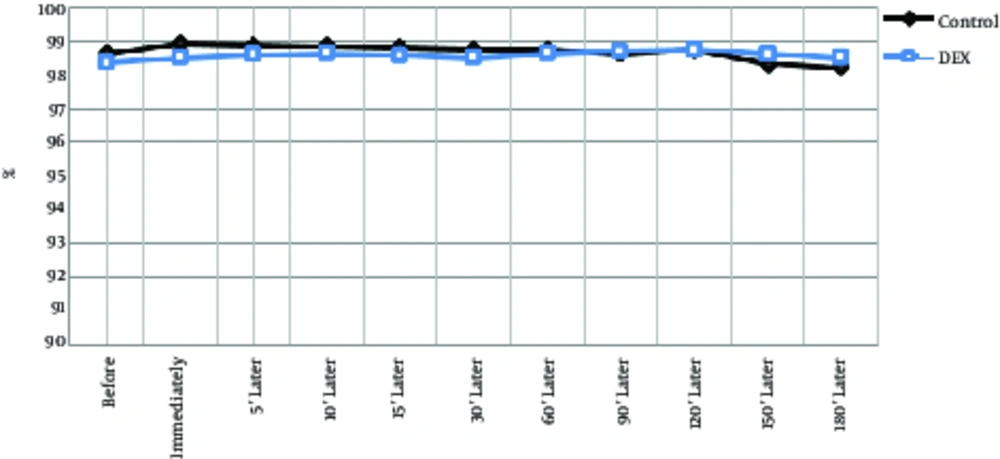
The 2 groups did not show significant differences in the number of breaths per minute or as an intra-group (P > 0.05), although the average respiratory rate per minute was lower in the dexmedetomidine group. Temperature decrease of the tympanic curtain was significantly lower in the DEX group at 10 minutes and 60 minutes after surgery than in the control group (Table 2, Figure 4, P < 0.001). Body temperature range was between 37.5 and 36.5°C in both groups and the lowest temperature of the tympan was 180 per minutes. In intra-group comparisons, in both groups, body temperature was decreased by an average of less than 0.8 degrees Celsius, which was significantly reduced compared to the preoperative time and during operation in most cases (Table 2, Figure 5, P < 0.05). Arterial oxygen saturation in both groups before and after operation was more than 97%; no difference was observed between the 2 groups. In intra-group comparisons, there was no significant difference in arterial oxygen saturation between groups compared to before the operation (Table 2, Figure 6).
Before the surgery, there was no significant difference between the 2 groups before and after surgery, while there was a significant difference between the two groups during and after operation. Comparison of mean values showed that shivering was less in the dexmedetomidine group than in the control group (Table 3).
Results of T-Test Analysis of Shivering Intensity Before, During and After Surgery
5. Discussion
The purpose of this study was to investigate the effect of dexmedetomidine prescription on the incidence of shivering during surgery in a spinal anesthetic technique under elective orthopedic surgery of the lower limb in addicted patients. The results showed that prescription of dexmedetomidine versus placebo significantly inhibited shivering in patients and decreased the incidence of shivering to 1/3 of the placebo group. Based on the literature review, clinical trials have not been conducted to evaluate the effect of anti-shivering of dexmedetomidine in addicts during anesthesia and existing studies have been conducted in non-addicted individuals. The findings showed that the use of dexmedetomidine in spinal anesthesia reduced shivering during and after surgery. The results of the study coincide was in line with similar studies in both spinal anesthesia and general anesthesia. For example, in the study of Bicer et al. (2006), dexmedetomidine injection was associated with an incidence of 6 cases of shivering (15%) during general anesthesia, while this rate was reported in the control group at 55% (15). Rahimzadeh et al. (2015), mentioned that dexmedetomidine in patients with spinal operation is associated with lower postoperative pain score and intraoperative bleeding. Hemodynamic effects are significantly better in patients that received dexmedetomidine (16).
Bajwa et al. (2012), reported that intravenous dexmedetomidine injection (1 mg/kg) during general anesthesia caused shivering in only 5% versus 42.5% in the control group, which was statistically significant (9). In research conducted by Karaman et al. (2013), intravenous dexmedetomidine injection (1 mg/kg) in laparoscopic women led to significant decrease in shivering as compared to the placebo group, which in the dexmedetomidine group and the placebo group was 10% and 46.7%, respectively (17). Moaward et al. (2015), reported that shivering in the dexmedetomidine group and in the control group was 15% and 57%, respectively in patients undergoing prostatectomy by TURP (18). In the study of Arora et al. (2014), the incidence of shivering in the tramadol group (1 mg/kg) was 6.6% and in the dexmedetomidine group (0.5 mg/kg) was 10% during spinal anesthesia, which was not significantly different (19).
Hu et al. (2016) reported shivering was 23.3% during spinal anesthesia and dexmedetomidine (1 mg/kg) and was 80% in the normal saline control group (20). In a study by Usta et al. (2011), regarding spinal anesthesia, dexmedetomidine significantly reduced the incidence of shivering (10% versus 56.7%) without significant difference in incidence of hypothermia compared with the placebo (70% versus 66.7%) (21). The exact cause of shivering during spinal anesthesia is not clear. In spinal anesthesia, areas below the block do not suffer from vasospasm due to sympathetic inhibition; therefore, vascular contraction and consequently shivering occur mainly in the upper regions of the spinal block. These areas affect the central temperature of the body. In this disorder, setting the central body temperature along with decreasing the threshold of shiver during spinal anesthesia, as well as exchanging body temperature with the surrounding environment, can be an effective cause for shivering during spinal anesthesia (1). Dexmedetomidine reduces the temperature threshold for shivering and can be effective in the control of shivering mechanism; in other words, shivering results in lower doses of dexmedetomidine injection. In the study of Doufas et al. (2003), the incidence of shivering was decreased from 36.7 ± 0.3 in the control group to 36 ± 0.5°C (22). In addition, in another study, although body temperature in dexmedetomidine recipients reached to 35.5 degrees, that did not significantly differ from the control group, however, the incidence of shivering was significantly lower (16), which could justify this hypothesis. In addition, the effect of agonist alpha-2 and inhibition of vasospasm of dexmedetomidine can be other mechanisms involved in inhibiting shivering (23).
Considering the effects of dexmedetomidine is very important on hemodynamic. Generally, in the present study, dexmedetomidine was not associated with hemodynamic impairment, and changes in blood pressure, body temperature, respiratory rate, and heart rate of the recipients were within the normal range, and only in transient time, there was a significant difference with the placebo group except for respiratory rate. Also, hemodynamic changes in the dexmedetomidine recipients was generally decreased or recovered more slowly. These findings are consistent with the results of other studies (19, 20, 22). On the other hand, hemodynamic symptoms with dexmedetomidine injections are less likely to fluctuate, and the changes are more reliable and predictable. In addition, the percentage of arterial oxygen saturation was not significantly different in the patients with dexmedetomidine compared to the control group, it can be noted that dexmedetomidine did not increase oxygen consumption; in other words, in the case of shivering, which is associated with an increase in oxygen consumption, dexmedetomidine does not have an additional effect on oxygen consumption and do not prevent increasing heart rate and hypertension, according to the current findings (no hypotension and bradycardia in the use of dexmedetomidine in this study). In this study, sleep deprivation and mouth dryness were significantly increased with dexmedetomidine, and did not increase the incidence of nausea, vomiting, and itching, which confirms the results of past studies. For example, in the study by Bajwa et al. (2012), mouth dryness in the dexmedetomidine group was greater than in the control group; this drug had a protective effect on nausea, vomiting, and headache (9). In the findings of Usta et al. (2011), allergy, hypotension, nausea, vomiting, headache, urinary impairment, and back pain in the spinal block were not significantly different from with and without the use of dexmedetomidine (21). Intradental injection of dexmedetomidine in spinal anesthesia for TURP surgery was not significantly different from the control group for nausea and vomiting, however, it was associated with hypotension (20.5% vs. 5%) and bradycardia (15% vs. 2.5%) compared to the placebo (18).
In addition to inhibit shivering, dexmedetomidine has other advantages as follows: positive effects on the amount of anesthesia during anesthesia (9, 19, 24), analgesia, and sedation, especially for controlling the symptoms of tracheal syndrome and detoxification of the substance (25), and consequently reducing the need for opiate during anesthesia (24), reducing the need for intubation (15, 17), increased wakefulness and alertness (15), shortening the duration of shivering (8), and no significant effect on the respiratory system, such as respiratory suppression (11, 26, 27). Similar to our findings, in the current study, there was no negative effect on arterial blood oxygen saturation and respiratory rate, which is the other benefits of dexmedetomidine, especially in opium addicted patients with an overdose, which can be a factor in weakening the respiratory system (28-30).
5.1. Research Limitations
The limitation of this study was the non-diversion of abused drugs to eliminate the adverse effect of drug abuse on the effects of anti-shivering of dexmedetomidine and its side effects, as well as the investigation of spinal anesthesia and not general anesthesia, which can affect the outcome (31). In addition, the lack of measuring the temperature of the surgical room and the recovery room, which may have a potential effect on incidence of shivering, (5) is another limitation that can be addressed in future studies.
5.2. Conclusions
In the end, the findings of this clinical trial study, in opiate addicted patients under spinal anesthesia, showed that the prescription of dexmedetomidine reduced the incidence of shivering in the lower limb and generally, had no adverse effect on hemodynamic parameters and was associated in patients with minimal clinical complications such as nausea, vomiting, and itching. It can be used as an effective and safe drug in people with drug abuse to control shivering during surgery.