1. Background
Rhinoplasty is still one of the most popular surgical procedures in the world (1) and bleeding during operation through the blood rich nasal mucosa obscures surgical field visibility and sometimes results in suboptimal surgical outcome. Application of the controlled hypotension improves operative field visibility and decreases the duration of surgery (2), total blood loss (3, 4), and rate of postoperative edema and ecchymosis (2, 5).
Many different drugs are tried to achieve controlled hypotension conventionally defined as a reduction of the systolic blood pressure (BP) to 80 - 90 mmHg, a reduction of mean arterial pressure (MAP) to 50 - 65 mmHg or a 30% reduction of baseline MAP (6, 7).
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist (selectivity ratio for α2: α1 is 1600:1) (8). The sympatholytic effect of this α2 agonist made it attractive to be used as a hypotensive drug (9, 10) during the surgery. It results in a decrease in heart rate (HR) and cardiac output accordingly (11), whereas no decrease in stroke volume occurs unless the plasma concentrates reaches above 5.1 µg/mL (12). This drug also has sedative (12), amnesic, anxiolytic, hypnotic, and analgesic (13, 14) effects with minimal changes in respiratory variables (12, 15). Furthermore, it reduces postoperative nausea (13), vomiting (16), and shivering (17, 18). It also reduces delirium in patients after cardiac surgery (19).
Magnesium sulfate is studied as a hypotensive agent for several years in different surgical procedures (20-22). It decreases anesthetic, analgesic, and muscle relaxant (20) requirements as well as the incidence of nausea, vomiting, and shivering (21-23). Magnesium affects the regulation of sympathetic tone and BP through blocking N-type and partially L-type calcium channels, and as a result inhibits norepinephrine release (24). Magnesium acts as an N-methyl-D-aspartate (NMDA) receptor antagonist (25); therefore, it reduces analgesic and anesthetic requirements.
Although previous studies demonstrated the ability of the above mentioned drugs to provide controlled hypotension during operation, few studies compared them together specifically in rhinoplasty. The similar clinical characteristics of dexmedetomidine and magnesium sulfate beside the stable hemodynamic response to anesthesia and a significant decrease in HR following the administration of dexmedetomidine led to the design of the current study to recommend a more effective drug with fewer side effects. The current study aimed at comparing the efficacy of dexmedetomidine and magnesium sulfate to control BP during rhinoplasty and the resultant effects on the quality of surgical field in terms of bleeding and visibility. Recovery profile and complications were also compared.
2. Materials and Methods
The current randomized, prospective, double-blind study was conducted at a university hospital (Firoozgar Hospital affiliated to Iran University of Medical Sciences, Tehran, Iran), from May 2016 to February 2017. After obtaining the study protocol approval from the Ethics Committee of the university (code: IR. IUMS.REC 1395.138340) as well as informed consent from the subjects, 60 patients aged 18 to 50 years with ASA (American society of anesthesiologists) class I who were candidates for rhinoplasty were enrolled. Patients were randomly divided into 2 groups to receive either dexmedetomidine (Dex) or magnesium sulfate (Mg) as hypotensive agent during the surgery. Patients with revision rhinoplasty, known allergy to the study drugs, opioid abuse, bleeding disorder, and the ones using contraceptives were excluded from the study.
In the operating room, electrocardiogram (ECG), BP (systolic, diastolic, MAP), HR, peripheral oxygen saturation (SPO2), end-tidal carbon dioxide (ETCO2), and bispectral index (BISPECTRAL VISTA monitoring system; Covedian company, USA) were monitored. Neuromuscular blockade was also measured with TOF-Watch (TOF watch SX, Organon, Ireland). A crystalloid solution at a rate of 4 mL/kg/hour was started and continued through the surgery. In the DEX group, a loading dose of 1 µg/kg dexmedetomidine (Precedex, 200 µg/2mL, Hospira , USA) diluted in 50 mL of saline 0.9% was infused intravenously in 10 minutes before induction of anesthesia followed by continuous infusion of 0.4-0.6 µg/kg/hour during the maintenance of anesthesia. In the Mg group, a loading dose of 40 mg/kg of magnesium sulfate (50% solution, Pasteur Institute of Iran) diluted in 50 mL of 0.9% saline, was infused intravenously in 10 minutes before induction of anesthesia followed by continuous infusion of 10-15 mg/kg/hour during the operation. Anesthetic management as well as data collection and recording were performed by the same anesthesiologist blinded to the drugs applied to the groups. Both the study drugs were prepared by another person in a way that the rate of infusion/ body weight was similar in both groups. In order to exclude interpersonal variation in evaluation of surgical field, all the operations were performed by the same surgeon who also evaluated the surgical field.
Patients were preoxygenated with 100% oxygen and premedicated with 3 µg/kg of fentanyl. Anesthesia was induced with propofol 2.5 mg/kg and cisatracurium 0.15 mg/kg. Tracheal intubation was performed when post tetanic stimulation was zero. Oropharyngeal pack was used and patients were positioned in 20° reverse Trendelenburg. Anesthesia was maintained by the inspiration of 0.8% - 1.5% isoflurane at fresh gas flow with 50% nitrous oxide/oxygen mixture to keep bispectral index (BIS) values in a range of 40 - 60. Muscle relaxation was maintained with intermittent bolus administration of cisatracurium 0.02 mg/kg when more than 2 twitches were depicted by TOF watch. Patients were mechanically ventilated; respiratory rate and tidal volume were adjusted to provide a SpO2 level of more than 95% and end-tidal carbon dioxide level of 30 - 35 mmHg.
Five to ten milliliters of a solution containing 2% lidocaine and epinephrine 1:100,000 were administered locally in subperichondrial and supraperiosteal planes. Adrenaline cottonoids 1:1000 were also used for mucosal decongestant purposes at surgical sites.
In both groups, the goal was to achieve a mean arterial pressure of 60-70 mmHg. Isoflurane was adjusted to keep BIS values in the range of 40-60. If BP increased higher than desired level and HR also increased to more than 20% of preoperative value in spite of acceptable BIS values, 1 µg/kg of fentanyl was administered intravenously to treat inadequate analgesia. If only BP was higher than desired level, a bolus of nitroglycerine 50µg was administered intravenously. If BP decreased lower than the desired level, the rate of drug infusion was reduced in the current study. If BP did not reach the desired value, a bolus of ephedrine 5 mg was administered intravenously. In case of occurrence of bradycardia, if it was accompanied by a MAP less than 55 mmHg, a bolus of atropine 10 µg/kg was administered intravenously, but if MAP was in the acceptable range, infusion of the drug was discontinued and atropine administration was delayed till the HR got back to its normal values. The number of patients received fentanyl, nitroglycerin, atropine, or ephedrine as well as the total doses required in each group were recorded.
A bolus of alfentanil 8 µ/kg was administered before the performance of osteotomy. All the drugs were discontinued 10 minutes before the end of operation. Muscle relaxation was reversed with neostigmine 40 µg/kg and atropine 20µg/kg at the 4th twitch of TOF count. Patients were extubated when they were awake, TOF ratio more than 90%, and then transferred to the Post Anesthesia Care Unit (PACU).
HR, BP (systolic, diastolic, and MAP), and BIS values were recorded on arrival to the operating room, before induction of anesthesia, after intubation, and then 5, 10, and 15 minutes after intubation and every 15 minutes thereafter. TOF and end-tidal CO2 values were added to the abovementioned recordings after induction of anesthesia. The data were also recorded 2 minutes after epinephrine infiltration, at the end of surgery, after extubation, and every 15 minutes during the PACU stay. Isoflurane inhalation by fresh gas flow was recorded every 15 minutes and times required to repeat muscle relaxant administration were recorded.
The number of patients received fentanyl, nitroglycerine, atropine, or ephedrine as well as mean dose requirement in each group were recorded. Time of discontinuation of the anesthetic drugs and tracheal extubation (named the extubation time) was recorded as well.
Surgical field was assessed by the surgeon in terms of bleeding and visibility using a 6-option Liker-scale scale adapted from Fromme el al. (26): 0 = no bleeding; 1 = minor bleeding, but no aspiration required; 2 = minor bleeding, aspiration required; 3 = minor bleeding, frequent aspiration required; 4 = moderate bleeding, visible only with aspiration; 5 = severe bleeding, continuous aspiration required. Surgeon’s satisfaction with the operative field was rated using a 4 -option Likert scale at the end of surgery: 1 = bad, 2 = moderate, 3 = good, and 4 = excellent.
Postoperative sedation was evaluated using the Ramsay sedation score (27) at 15, 30, 45, and 60 minutes after tracheal extubation. Postoperative recovery was assessed by the modified Aldrete score (28), and time needed to reach score ≥ 9 was defined as the recovery period. Adverse effects such as bradycardia, shivering, nausea, and vomiting were recorded.
2.1. Statistical Analysis
Data were analyzed using IBM SPSS version 24.0 (Armonk, NY: IBM Corp). Normal distribution of data was tested by the Kolmogorov-Smirnov test. The student t test and the Mann-Whitney U test were used to compare numerical variables; Chi-square and the Fisher exact tests were used to compare categorical variables between the 2 study groups. MAP and HR were compared between the study groups using 2-way analysis of variance for repeated measurements. Results throughout the text, tables, and figures are expressed as mean ± SD. Two-sided P value < 0.05 was considered statically significant. Sample size was estimated based on the surgeon’s satisfaction score derived from the pilot study on 20 patients (10 in each group). For instance, surgeon’s satisfaction scores 1 - 2 were categorized as “low satisfaction” and scores 3 - 4 were categorized as ‘high satisfaction”. High satisfaction rates in the Dex and Mg groups were 60% and 20%, respectively. Considering type 1 error 0.05 and type 2 error 0.2, a minimum of 28 patients were needed in each group.
3. Results
A total of 60 patients were enrolled in the study of which 57 completed the study. Two patients in the Dex group were excluded, 1 because of refusing to participate in the study and the other due to drug abuse. One patient in the Mg group had hypothyroidism discovered in the operating room. Patients in both groups were matched by age, weight, gender (Table 1), duration of surgery, and isoflurane consumption (Table 2). Patients in the Dex group required more frequent administration of cisatracurium (P = 0.004).
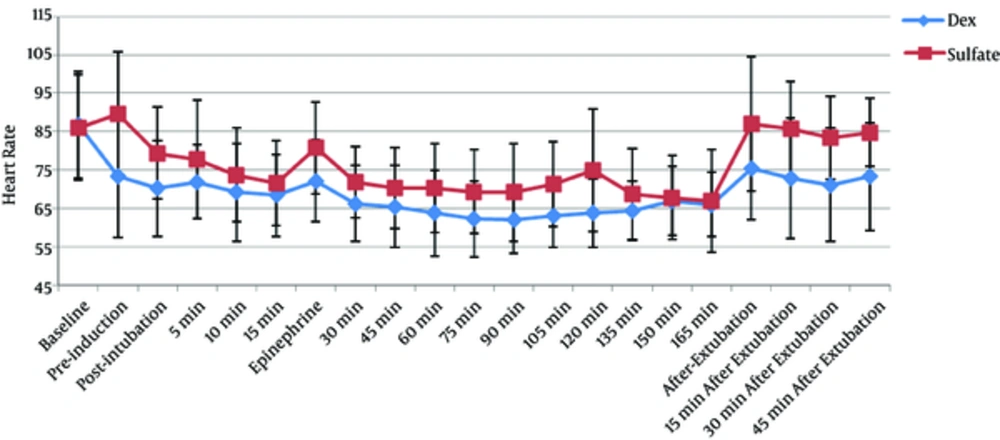
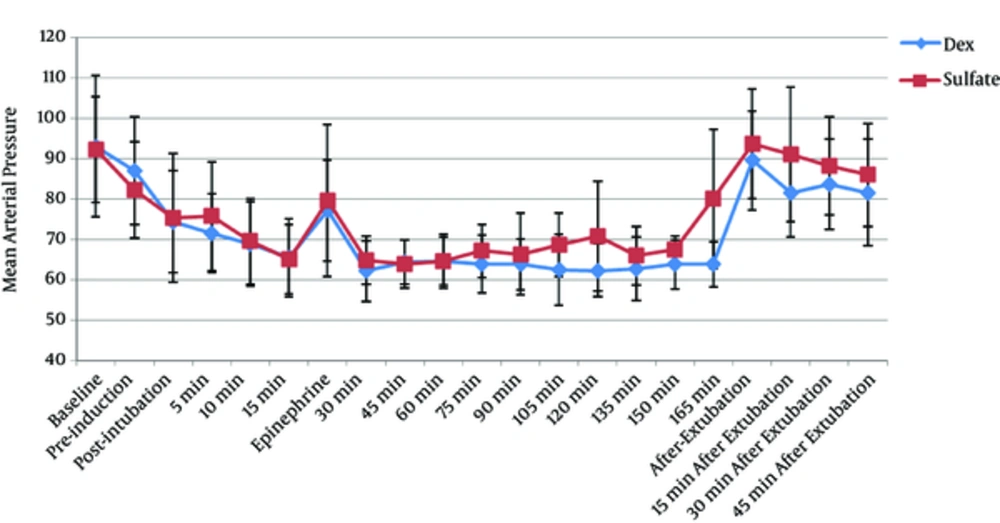
MAP was achieved within the desired range in both groups. There was no significant difference between the 2 groups in terms of the mean baseline HR and MAP. HR during operation (Figure 1) was significantly lower in the Dex group (P < 0.001), compared with that of the Mg group. Although mean MAP during operation (Figure 2) in the Dex and Mg groups was 68.21 ± 3.52 and 70.58 ± 5.35 mmHg, respectively, the difference was statistically insignificant (P = 0.054). MAP after epinephrine administration was also similar in both the study groups.
MAP and HR values at tracheal extubation and during the stay at PACU were higher in the Mg group, compared with those of the Dex group (P = 0.018 and P < 0.001, respectively).
More patients required fentanyl (P < 0.001) and nitroglycerin (P < 0.001) in the Mg group than the Dex group. Means of fentanyl dose (P < 0.005) and nitroglycerine dose (P < 0.001) required in the Mg group were higher as well (Table 2). None of the patients in the study groups required ephedrine administration. Bradycardia occurred in 7 patients in the Dex group and 1 patient in the Mg group. Five patients in the Dex group received atropine. The differences in the incidence of bradycardia and atropine requirement were significant between the groups (P = 0.011 and P = 0.023, respectively) (Table 2). Bleeding score (Table 3) was significantly lower (P < 0.001), and surgeon’s satisfaction score was significantly higher (P < 0.001) in the Dex group than in the Mg group.
Extubation time was similar in both the study groups. The Ramsay sedation score (Table 4) at 15, 30, 45, and 60 minutes was significantly higher in the Dex group, compared with that of the Mg group (P < 0.001). Time to reach Aldrete score ≥ 9 was also significantly higher in the Dex group, compared with the Mg group (P < 0.001) (Table 4). Two patients in the Mg group had nausea and vomiting and 1 patient in the same group had shivering, but the difference between the groups was insignificant.
| Dex Group (N = 28) | Mg Group (N = 29) | P Value | |
|---|---|---|---|
| Age, year | 26.28 ± 9.48 | 25.55 ± 7.1 | 0.682 |
| Gender, female/male | 21.7 | 25.4 | 0.331 |
| Weight, kg | 66.42 ± 12.95 | 68 ± 10.5 | 0.144 |
Data are expressed as mean ± standard deviation (SD) or number of patients
| Dex Group (N = 28) | Mg Group (N = 29) | P Value | |
|---|---|---|---|
| Duration of surgery, min | 144 ± 40.8 | 133.2 ± 45.6 | 0.35 |
| Isoflurane dose (%) | 0.817 ± 0.34 | 0.95 ± 0.18 | 0.074 |
| Times of intermittent cisatracurium required | 1.71 ± 1.27 | 0.82 ± 0.84 | 0.004 |
| Nitroglycerin requirement, µg | 8.92 ± 38.61 | 134.48 ± 165.88 | < 0.001 |
| Nitroglycerin requirement, no. of pts. | 3 | 15 | < 0.001 |
| Fentanyl requirement, µ/(kg) | 0.14 ± 0.44 | 0.62 ± 0.14 | 0.005 |
| Fentanyl requirement, no. of pts. | 2 | 13 | < 0.001 |
| Atropine requirement, no. of pts. | 5 | 0 | 0.023 |
| Extubation time, min | 14.1 ± 5.61 | 12.41 ± 3.92 | 0.27 |
| Nausea, no. of pts. | 0 | 2 | 0.25 |
| shivering, no. of pts. | 0 | 1 | 0.51 |
Data are expressed as mean ± SD or number of patients, no. of pts.
Data are expressed as number of patients and, in parenthesis, the percentage in each group
a Bleeding score: 0 = no bleeding; 1 = slight bleeding, no aspiration required; 2 = minor bleeding, aspiration required; 3 = minor bleeding, frequent aspiration required; 4 = moderate bleeding, visible only with aspiration; 5 = severe bleeding, continuous aspiration required
b Surgeon’s satisfaction score: 1 = bad; 2 = moderate, 3 = good; 4 = excellent
| Dex Group (N = 28) | Mg Group (N = 29) | P Value | |
|---|---|---|---|
| Time to reach modified alderete score (28) ≥ 9, min | 22.53 ± 3.56 | 15 ± 2.89 | < 0.001 |
| The ramsey scorea at 15 min after surgery | 4.71 ± 0.46 | 2.4 ± 0.5 | < 0.001 |
| At 30 min after surgery | 4.32 ± 0.66 | 2.24 ± 0.43 | < 0.001 |
| At 45 min after surgery | 4.17 ± 0.66 | 2.1 ± 0.3 | < 0.001 |
| At 60 min after surgery | 3.07 ± 0.71 | 2 ± 0 | < 0.001 |
Data are expressed as mean ± SD or number of patients
aThe Ramsay sedation score: 1 = anxious, agitated or restless; 2 = cooperative, oriented and tranquil; 3 = responsive to commands; 4 = asleep, but with brisk response to light glabellar tap or light auditory stimulus; 5 = asleep, sluggish response to glabellar tap or auditory stimulus; 6 = asleep, no response
4. Discussion
According to the conventional standards of controlled hypotension employed in many studies (6, 7), it is intended to maintain MAP at 50 - 60 mmHg. Edram et al., (29) assessed regional cerebral oxygen saturation using infrared spectroscopy during controlled hypotension in patients undergoing rhinoplasty. They reported that when MAP decreased to 50 - 60 mmHg, cerebral desaturation occurred in 10% of the patients. Based on their results, to prevent the occurrence of undistinguished cerebral hypoxia, it was decided to maintain the MAP in 60 - 70 mmHg in the current study.
The current study results suggest that controlled hypotension was achieved in both groups and despite the lower mean values of MAP in the Dex group, the difference was not statistically significant between the study groups; but the operative field condition was more favorable in the Dex group than the Mg group in terms of bleeding and visibility. Moreover, BP control was easier in the Dex group, that is, the number of patients that required nitroglycerine or fentanyl administration was lower in the Dex group. Furthermore, mean dose of nitroglycerine and fentanyl required in the Dex group was lower than those of the Mg group.
In several studies, dexmedetomidine was compared with esmolol or remifentanil as a hypotensive drug, and the results were inconsistent. In some studies, dexmedetomidine produced lower HRs and BP as well as better surgical field condition, compared with esmolol (30, 31), whereas in other studies these effects of dexmedetomidine were similar to those of esmolol (32). It was also demonstrated in some studies that dexmedetomidine was as effective as remifentanil to produce controlled hypotension with similar hemodynamic properties (33, 34), but in another study, it was concluded that dexmedetomidine was less effective than remifentanil to achieve controlled hypotension and good surgical field exposure (35). Magnesium was also compared with remifentanil in 2 studies (21, 36). Both drugs were similar in terms of providing controlled hypotension; lower HRs and MAP were reported with magnesium in a study, while the other one (36) reported similar hemodynamic properties in the study groups.
The current study results were consistent with previous studies on dexmedetomidine. Bayram et al., (37) compared the hypotensive effects of dexmedetomidine and magnesium sulfate during functional endoscopic sinus surgery. They reported lower bleeding and higher surgeon’s satisfaction scores with dexmedetomidine administration in addition to significantly lower HRs and MAP during the operation using this drug compared with those of magnesium sulfate. They used the same drug dose regimens as used in the current study.
In the current study, patients in the Dex group had lower HRs than the ones in the Mg group during the operation, which may explain the better surgical field condition in the Dex group. Some studies (38, 39) reported that by decreasing the HR, a better operative field condition was provided. Moreover, as Sieskiewicz et al. found (40), to provide a better surgical condition, there was no need to decrease MAP to the risky low levels if HR was maintained as low as 60 beat/minute. Dexmedetomidine induces bradycardia through its action on α2-agonist receptors (8). Although magnesium reduces HR (20) probably through inhibition of norepinephrine release (24), it seems that the decrease in HR is more prominent with dexmedetomidine rather than magnesium. In the study by Byram et al. (37), significantly slower HRs during anesthesia in the dexmedetomidine group was also reported.
Lower HRs in the Dex group might be one of the reasons that better surgical field condition was achieved in this group. However, bradycardia occurred in 7 patients in the Dex group, 5 of which needed atropine administration, while bradycardia occurred just in 1 patient of Mg group and no atropine was required in this group. In the study by Byram et al. (37), although the incidence of bradycardia (i e, HR decrease more than 20% of initial rate) was similar in both groups, it occurred in 4 patients in the Dex group versus 1 patient in the Mg group. Moreover, there are some case reports of cardiac arrest (41-43) following dexmedetomidine administration, which should be considered when choosing this drug as a hypotensive agent during operation.
In the current study, bleeding score was lower and surgeon’s satisfaction score was higher in the Dex group rather than those of the Mg group. Besides the decrease in BP and HR by dexmedetomidine administration, peripheral vasoconstriction might be another reason for less bleeding and better quality of surgical field provided by this drug. Lawrence et al., (44) reported that dexmedetomidine decreased organ blood flow in dogs, with the largest decrease in the skin (up to 90%). This vasoconstriction may be achieved through the known vasoconstrictive effect of dexmedetomidine on postsynaptic α2 adrenoceptors located in peripheral blood vessels (45).
In the current study, less opioid was required in the DEX group than the Mg group. Many studies showed that dexmedetomidine reduced opioid (9, 10, 13, 14) requirements during and after the operation. Dexmedetomidine exerts its analgesic effect through α2 receptors in the locus coeruleus and spinal cord (46). Magnesium acts as NMDA receptor antagonist (25). Different results were reported about the analgesic effect of magnesium. In the study by Cizmeci et al. (47), magnesium did not reduce analgesic requirements during anesthesia, whereas in a meta-analysis by Albercht E et al. (48), it was concluded that magnesium sulfate reduced postoperative pain. Results of the current study showed that dexmedetomidine administration decreased opioid requirement with a greater extent than magnesium administration. Though it seems that dexmedetomidine has stronger analgesic effect than magnesium.
Previous studies showed that magnesium administration enhanced neuromuscular blocking action of muscle relaxants (47-49). Magnesium acts as a calcium channel blocker at presynaptic nerve terminals, thus it causes a decrease in presynaptic acetylcholine release at the motor endplate and has some advantages for general and neuraxial anesthesia (50, 51). The results of the current study were consistent with those of previous studies. The requirement for cisatracurium was less in the Mg group than the Dex group, while duration of anesthesia was the same in the 2 groups.
In the current study, patients in Dex group were more sedated at all recording times in the PACU and the time duration to reach modified Aldrete score ≥ 9 was longer compared with those of the Mg group.
Byram et al. (37) reported contradictory results. In their study, the duration to reach modified Aldrete score ≥ 9 was shorter with dexmedetomidine rather than magnesium. Magnesium decreased anesthetic requirements in some studies (20). In the studies comparing intravenous dexmedetomidine and intravenous remifentanil administration as a hypotensive agent during operation, it was observed that patients receiving dexmedetomidine were more sedated (32) (using different scoring system), and the time needed to reach modified Aldrete score ≥ 9 was longer (33) than those of the ones that received remifentanil. Similarly, sedation score was higher when dexmedetomidine was administered to induce hypotension during functional endoscopic sinus surgery, compared with that of esmolol (29).
4.1. Conclusion
Dexmedetomidine was more effective than magnesium to achieve controlled hypotension, and provide a favorable surgical field condition. However, this drug also comes with a heightened risk of induced bradycardia and prolonged sedation. These are 2 important points to consider when choosing this drug as a hypotensive agent during operation.