1. Introduction
Intrathecal (IT) baclofen therapy is a well-established treatment option for patients with refractory spasticity secondary to multiple sclerosis, stroke, traumatic brain injury, cerebral palsy, and spinal cord injury (1, 2). Patients with these conditions are implanted with a programmable pump that delivers a preset dose tailored to each patient’s symptoms (1-3). However, a recent study revealed that the pump failure/complication rate was approximately 35% over 12 years with many of these events related to catheter performance (4). Incremental improvements in the design of these devices and refinements in the implant technique have consistently increased catheter and pump survival (4-6). The introduction of the Ascenda (Medtronic, Inc.) catheter to the market is one such example (6). Approved for use in 2011 by the FDA, it was initially associated with a reduction of major complications in comparison to silicone catheters (6). Although the elasticity modulus for this reinforced catheter is improved, it can still be susceptible to extreme shear and strain forces generated in patients.
Kinking of the catheter and migration of the pump are recognized risk factors for catheter occlusion with previous generation of catheters (4, 5, 7-10). Other adverse effects associated with intrathecal drug delivery include spinal headache, as reported by Kurnutala et al. in 2015 (11). The twisting and occlusion of the catheter in ITB systems can also be described as Twidder’s syndrome; first coined in 1968, Twiddler’s syndrome described the malfunction of a pacemaker secondary to manipulation of the pulse generator by the patient (12). Specifically, voluntary or involuntary patient movement dislodges and tangles the leads around the generator stimulating the ipsilateral phrenic nerve (12). This is associated with diaphragamic pacing, abdominal pulsations, and cessation of ventricular pacing (12). The same principle of Twiddler’s syndrome can also been applied to other devices including programmable pumps where catheter occlusion obstructs outflow (4, 9, 13). In this report, we describe the first documented case of Twiddler’s syndrome in a patient with an Ascenda catheter resulting in acute baclofen withdrawal.
2. Case Presentation
A 32-year-old, man, with a medical history significant for severe spasticity secondary to spinal cord injury presented to the ED with signs and symptoms consistent with baclofen withdrawal 19 months after insertion of a programmable pump.Prior to intrathecal therapy, he was managed with oral baclofen, tizanidine and botulinum toxin injections. Despite involvement with physical therapy and stretching daily, he continued to experience severe spasms exacerbated with ambulation or when recumbent. A 37.5 mcg test injection of IT baclofen reduced his spasms and improved his range of motion. He consented for implantation of a permanent programmable pump that could deliver IT baclofen continuously. A tunneled Ascenda catheter was deployed into the IT space with the tip positioned over the T9 - T10 interspace under fluoroscopy. A 40 mL Synchomed II pump was fitted into the suprafascial right abdominal wall, anchored and connected to the pump segment that was secured to the catheter. There were no perioperative complications.
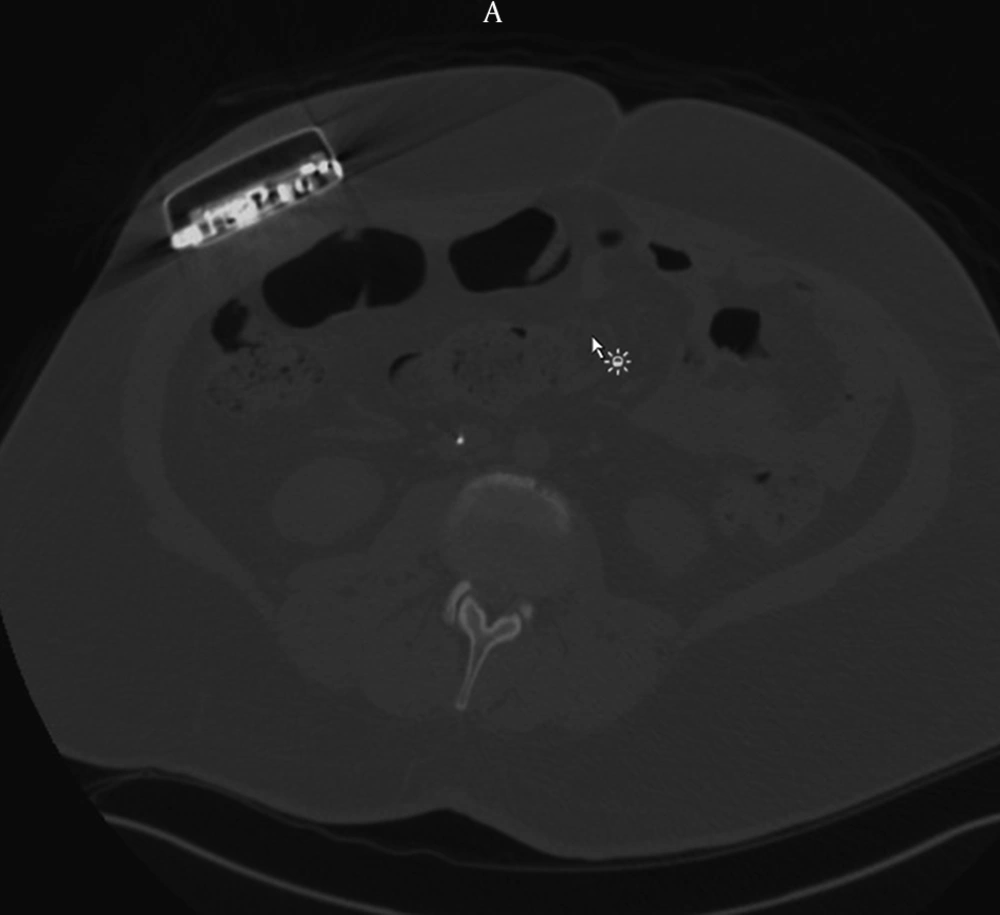
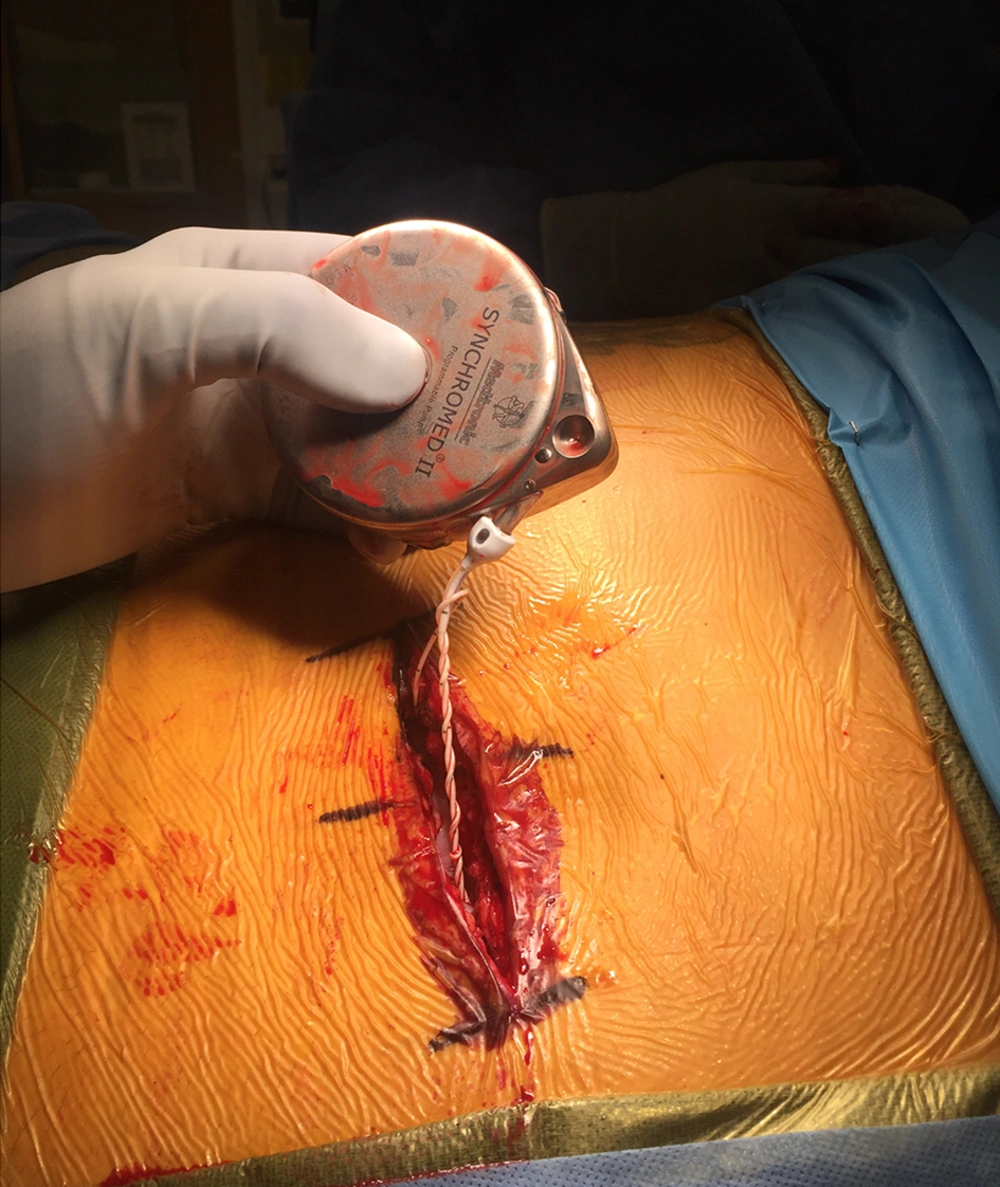
The patient reported marked relief in his spasticity until he presented 19 months later in baclofen withdrawal. Axial CT revealed that the pump had flipped within the abdominal pocket (Figure 1). He was taken to the OR for urgent pump revision. The incision was reopened and the scar tissue was cut until the pump was exposed and explanted from the pocket. The coiled catheter was dissected free (Figure 2).
The catheter was unwound and the side-port aspirated. CSF flowed easily. Careful examination of the catheter disclosed significant damage. It was decided to replace the pump segment. After the pump segment was secured, the side-port was aspirated again to verify flow. The incision was closed and the patient was awoken from anesthesia and discharged uneventfully. At follow-up, the patient reported resolution of his symptoms.
3. Discussion
To our knowledge, this is the first reported case of an Ascenda catheter occlusion in a patient with Twiddler’s sydrome. Prior to introduction of this device, catheter complications may have been more frequent. A review of previously reported cases of ITB system malfunction consistently identifies the catheter as the most vulnerable component of the ITB system (5, 9, 14). For example, a systematic review performed by Stetkarova et al. in 2010 reported that of the 558 complications in 1,362 pump implantations, 66% were related to catheter malfunctions (10).
Modern series suggest that the catheter related complications have subsided (3). Motta and Antonello reported only 1 patient out of 92 with a catheter related complication when the Ascenda was chosen (6). In patients implanted with a silicon catheter, 120 out of 416 experienced a complication. In a recent report from the implantable systems performance registry (ISPR), device survival probability for the SynchroMed II pump (used by our patient) is 99.9% at one year (4). By comparison, the Ascenda catheter has a 90% survival probability over one year (4). Long term survival data of the Ascenda included in this registry are not yet available (4). A pump that is continuously flipped in its pocket, which causes the catheter to wind on itself and obstruct flow (as is the case in the present patient), is cited as a very uncommon complication (9, 10, 13).
In this case, it is likely the fixation failed because the pump was not able to be adequately secured to the fascial wall due to depth limits related to excess adipose tissue. This, coupled with his ambulatory status compounded the risk of this event. Obese patients should be cautioned about this risk. Surgical technique modification, pump location or the continuous use of abdominal binders could mitigate the occurrence of this complication.