1. Background
Pain can become intractable and chronic when neural damage and inflammation are processed under disrupted psychosocial conditions. The transition from acute pain into intractable and chronic pain is actively modulated (plasticity) at the molecular to the network level since many neuromodulators invariably work for neuroplasticity of pain perception (1, 2).
Authors previously reported that stool consistency displays a significant association with the pain perception and anxiety status of healthy volunteers (3). Several researchers reported that stool consistency is profoundly linked to gut microbiome (GM) abundance and composition, enterotypes, and bacterial growth (4-7). In contrast, some studies show that constipation is deeply linked to microbial diversity and composition (8, 9). There is increasing evidence that changes in microbial diversity and composition are associated with several disease states including obesity and behavioral disorders. In the past few years, the human microbiome is recognized as a considerable contributor to human nutrition as well as health and disease (10). It was thus postulated that the GM dysbiosis might be associated with pain perception and anxiety states in healthy subjects. However, it is not clear if there is an association between GM and pain severity in patients with chronic pain in the authors’ previous study, since it was conducted on young healthy subjects.
The GM is known to influence host neuromodulatory, neurotransmission, and neuroimmune functions (11, 12). Since it was hypothesized that pathogenic bacteria indispensably work on neuroplasticity of pain perception in a maladaptive way, thereby exacerbating chronic pain, the current study aimed at investigating the relationship between stool form or constipation and pain severity in patients with chronic pain.
2. Methods
After receiving approval from the IRB (Aichi Medical University reference number: 12 - 067), a cross sectional survey was administered to a total of 365 patients with chronic pain that visited the pain center of Aichi Medical University Hospital to manage their chronic pain from March 2017 to April 2017. The demographics, medication, and course of pain in all patients were recorded on a regular basis. The exclusion criteria were digestive disease that may be cause constipation and diarrhea, stoma in situ, neurological diseases such as spinal cord injury and autonomic disturbance, or cognitive disease.
The participants were evaluated based on their usual stool consistency, constipation state, and degree of obesity and usual pain over a period of one week. The stool consistency was assessed by the Bristol stool form scale (BSFS). The BSFS is a graded visual scale of stool density from type 1 (hard to pass) to type 7 (the fluid kind). The relevance of this scale is that it shows the participant’s drawing stool shapes together with precise descriptions regarding form and consistency, and using easily recognizable examples (for example, in type 1, by an illustration of faces as separate balls, a description: “separate hard lumps, like nuts”). The stool types 1 and 2 (“sausage-shaped, but lumpy”) considered abnormally hard stools (designated as constipation symptoms), types 3, 4, and 5 are generally considered normal stool form, especially type 4 (“like a sausage or snake, smooth and soft”) is most common, and types 6 (“fluffy pieces with ragged edges, a mushy stool”) and 7 (“watery, no solid pieces”) are abnormally liquid stools (designated as diarrhea) (13). Constipation was rated with the Cleveland clinic constipation score (CCCS). This score is compatible with objective physiologic findings, provides standardized assessment of constipation, and is validated in clinical practice. CCCS consists of eight factors: frequency of bowel movements, difficulty (painful evacuation effort), completeness (incomplete evacuation), pain (abdominal pain), time (minutes in lavatory per attempt), assistance (type of assistance), failure (number of unsuccessful attempts of evacuation per 24 hours), and history (duration of constipation). The scoring of each factor ranges from 0 to 4 (with the exception of “type of assistance”, which is 0 to 2). Score ranges from 0 to 30, with 0 indicating normal and higher scores indicating more severity constipation (14, 15). The degree of obesity was assessed by using the body mass index (BMI). The scores of numerical rating scale (NRS) scores (0 indicates ‘no pain’ and 10 ‘the greatest pain possible’) were used to obtain the average severity of total pain over a period of one week.
2.1. Data Analyses
All data were analyzed with SPSS version 20 (IBM, New York, USA). Data were expressed as median and range, since each variable resulted in not only parametric but also non-parametric distribution. The participants were assigned into five groups according to the pain region (i e, low back and/or lower limb, whole body, neck and/or upper back and/or upper limb, head and/or face, chest and/or abdominal). First, G-power software was employed to determine the sample size for the current study. An effect size means the strength of correlation between two variables. In the magnitude of the effect size in correlation, 0.3 and 0.5 mean medium and large effect size, respectively. The sample size required a minimum of 60 subjects to show an effect size of 0.4 with a significance level of 0.05 (α = 0.05) and a power of 80% (β = 0.20) for each group; therefore, a total of more than 300 samples were needed for the study. Analysis of variance and the Fisher exact or the Kruskal-Wallis tests were performed for patients’ characteristics and medication where appropriate. The relationship among outcome measures was analyzed using Spearman correlation for bivariate regression analysis. Further analysis using a stepwise multiple linear regression analysis was performed to predict the pain severity of the independent variables (i e, gender, age, BMI, BSFS, CCCS). A P-value of < 0.05 was considered statistically significant.
3. Results
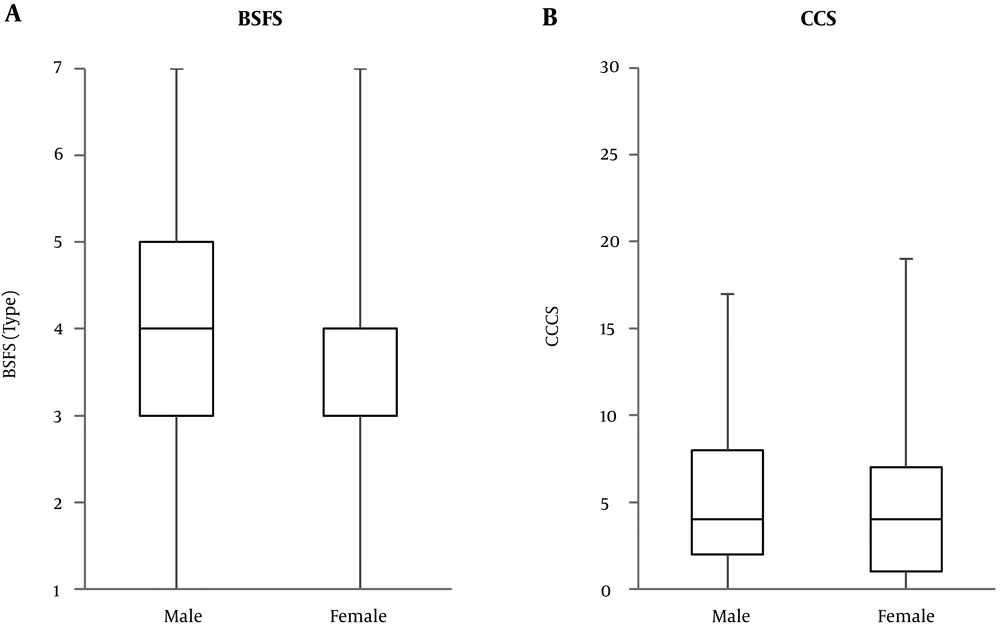
Three hundreds and three out of 365 patients with chronic pain completed the questionnaire. Their characteristics are presented in Table 1. No significant differences were observed in height (cm), weight (kg), BMI (kg/m2), BSFS, and CCCS among the pain regions. There were some differences in the gender ratio among the groups. However, BSFS and CCCS did not show gender differences (Figure 1). Patients of the low back and/or lower limb group were older. NRS score was statistically lower in the low back and/or lower limb and head and/or face groups than the whole body group. The BSFS showed a significant and negative association with age and BMI, but did not show association with the pain severity. On the other hand, CCCS showed a significant and positive association with the pain severity, but no association with age and BMI (Table 2).
| Total | Low Back/Lower Limb | Whole Body | Neck/Upper Back/Upper Limb | Head/Face | Chest/Abdominal | P Value | |
|---|---|---|---|---|---|---|---|
| No. (male: female) | 303 (121: 182) | 111 (53: 58) | 71 (24: 47) | 62 (27: 35) | 36 (6 : 30) | 15 (6: 9) | 0.014 |
| Age (y) | 57 (11 - 90) | 64* (11 - 87) | 52 (14 - 90) | 52 (18 - 86) | 56 (15 - 80) | 49 (15 - 84) | 0.012 |
| Height (cm) | 160 (131 - 184) | 160.0 (138 - 183) | 158.0 (147 - 182) | 164 (132 - 184) | 155 (146 - 171) | 158 (146 - 183) | 0.056 |
| Weight (kg) | 55.0 (32 - 111) | 58.0 (37 - 105) | 53.0 (32 - 90) | 55.7 (40 - 111) | 53.0 (36 - 86) | 58.0 (44 - 77) | 0.582 |
| BMI (kg/m2) | 21.7 (12.2 - 41.4) | 22.1 (14.5 - 41.4) | 20.8 (12.2 - 36.6) | 21.0 (15.8 - 36.2) | 21.0 (15.7 - 33.3) | 23.4 (17.6 - 26.1) | 0.203 |
| Pain severity (NRS) | 6 (0 - 10) | 5* (0 - 10) | 6 (2 - 10) | 6 (0 - 10) | 5* (1 - 10) | 6 (2 - 8) | 0.008 |
| BSFS | 4 (1 - 7) | 4 (1 - 7) | 4 (1 - 7) | 4 (1 - 6) | 4 (1 - 6) | 4 (2 - 5) | 0.681 |
| CCCS | 4 (0 - 19) | 4 (0 - 17) | 4 (0 - 15) | 4 (0 - 19) | 3 (0 - 12) | 2 (0 - 16) | 0.108 |
Abbreviations: BMI, body mass index; BSFS, the Bristol stool form scale; CCCS, the Cleveland clinic constipation score; NRS, numerical rating scale.
a Value: median (range).
b*, vs. Whole body: P < 0.05.
c Others (n = 8), Cancer Pain (n = 1), Postherpetic Pain (n = 1), Endometriosis (n = 1), Arteriosclerosis Obliterans (n = 1), Anus Pain (n = 1), Pudendal pain (n = 3).
| BMI | Pain Severity | BSFS | CCCS | |
|---|---|---|---|---|
| Age (y) | 0.184** | -0.015 | -0.116* | 0.052 |
| BMI (kg/m2) | -0.118* | -0.174** | -0.047 | |
| Pain severity | 0.014 | 0.227*** | ||
| BSFS | -0.175** |
Abbreviations: BMI, body mass index; BSFS, the Bristol stool form scale;CCCS, the Cleveland clinic constipation score.
a Value: correlation coefficient, * P < 0.05, ** P < 0.01, *** P < 0.001.
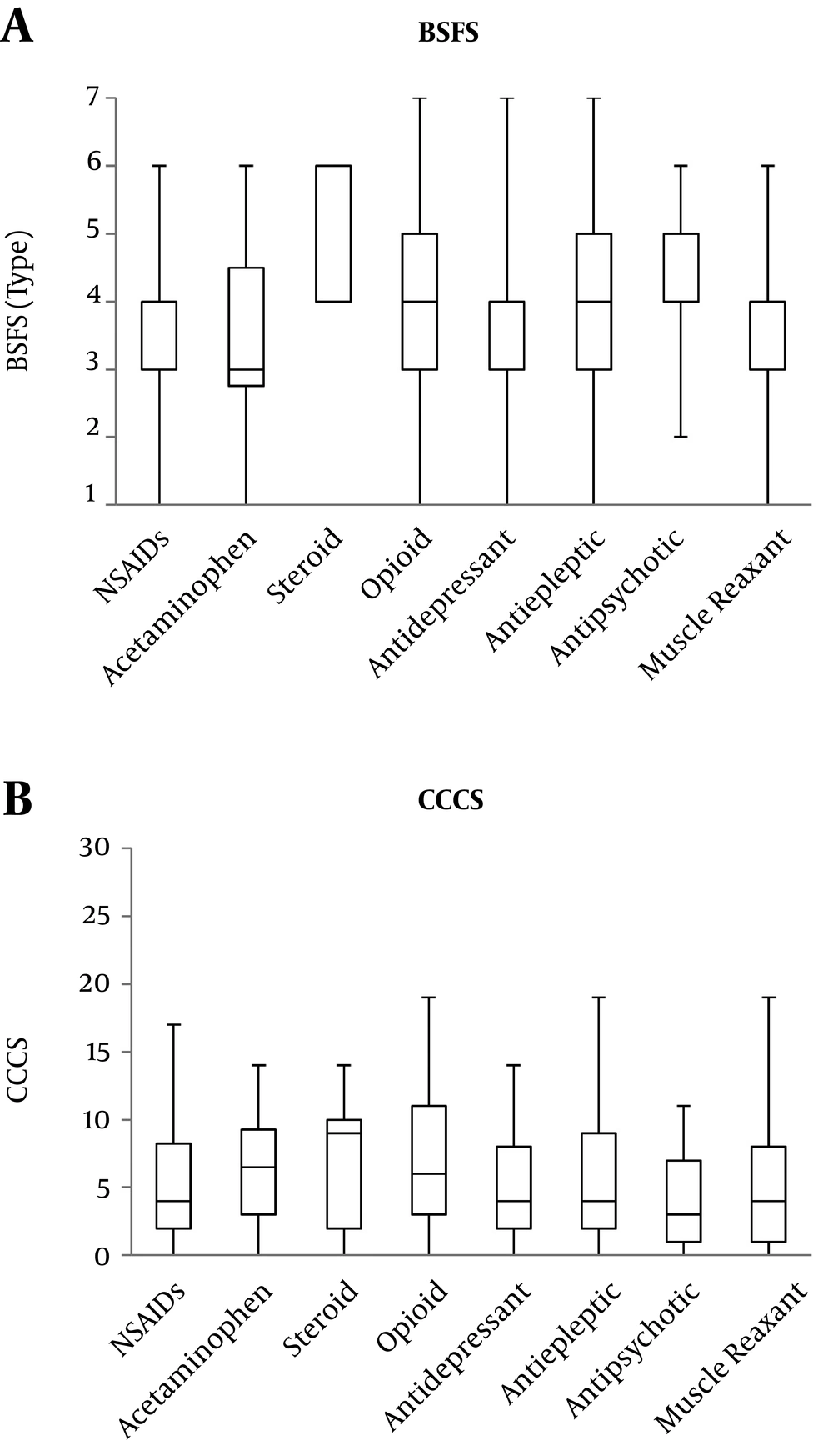
For medication, there were some differences of the prescribed ratio only in acetaminophen (Table 3). Furthermore, there were no significant differences in BSFS and CCCS (P = 0.183, P = 0.292) among medications (Figure 2).
| Low Back/Lower Limb | Whole Body | Neck/Upper Back/Upper Limb | Head/Face | Chest/Abdominal | P Value | |
|---|---|---|---|---|---|---|
| NSAIDs | 19 (17.1) | 13 (18.3) | 7 (11.3) | 5 (13.9) | 2 (13.3) | 0.808 |
| Acetaminophen | 2 (1.8) | 7 (9.9) | 1 (1.6) | 1 (2.8) | 0 (0) | 0.040* |
| Steroid | 1 (0.9) | 4 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0.062 |
| Opioid | 22 (19.8) | 21 (29.6) | 17 (27.4) | 5 (13.9) | 2 (13.3) | 0.234 |
| Antidepressant | 18 (16.2) | 8 (11.3) | 12 (19.4) | 8 (22.2) | 3 (20.0) | 0.599 |
| Antiepileptic | 32 (28.8) | 34 (47.9) | 22 (35.5) | 12(33.3) | 3 (20.0) | 0.433 |
| Antipsychotic | 1 (0.9) | 2 (2.8) | 2 (3.2) | 4 (11.1) | 1 (6.7) | 0.056 |
| Muscle relaxant | 9 (8.1) | 10 (14.1) | 10 (16.1) | 3 (8.3) | 0 (0) | 0.242 |
Abbreviation: NSAIDs: nonsteroidal anti-inflammatory drugs.
a Values are available No. (%).
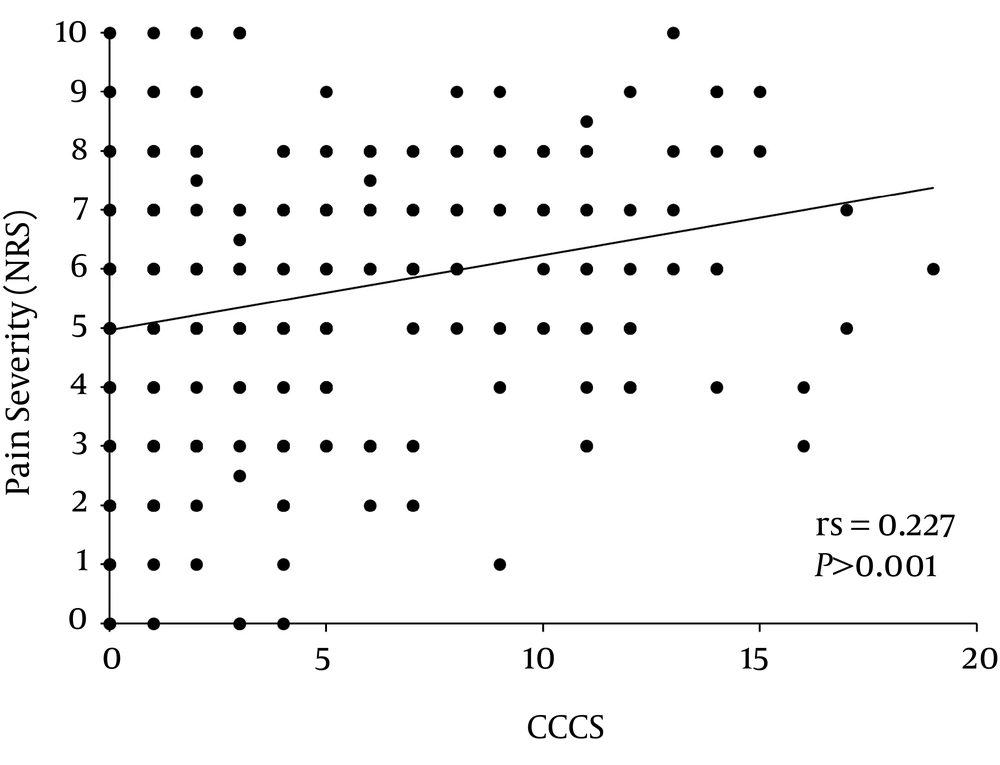
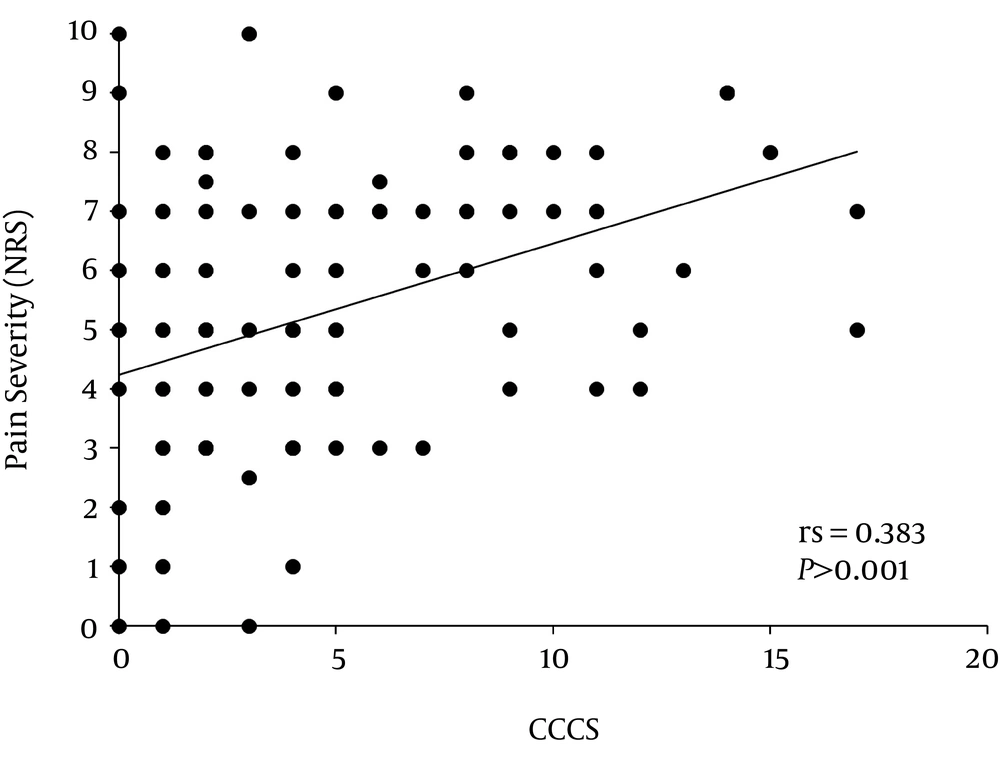
CCCS displayed a significant and positive association with the pain severity of the total patients and patients with low back and/or lower limb pain (Table 4, Figures 3 and 4). Simultaneous multiple linear regression analyses revealed that a predictor of pain severity was CCCS in the total patients and patients with low back and/or lower limb, and with whole body pain (Table 5). However, the BSFS showed no association with the pain severity at each pain region (Tables 4 and 5).
| Total | Low Back/Lower Limb | Whole Body | Neck/Upper Back/Upper Limb | Head/Face | Chest/Abdominal | |
|---|---|---|---|---|---|---|
| Age (y) | -0.015 | -0.102 | -0.077 | 0.259* | -0.053 | -0.183 |
| BMI (kg/m2) | -0.118* | -0.184 | 0.007 | -0.197 | 0.150 | -0.018 |
| BSFS | 0.014 | 0.062 | -0.081 | -0.073 | 0.027 | -0.127 |
| CCCS | 0.227*** | 0.383*** | 0.207 | 0.046 | 0.161 | 0.056 |
Abbreviations: BMI, body mass index; BSFS, the Bristol stool form scale; CCS, the Cleveland clinic constipation score.
a Value: correlation coefficient with pain severity, * P < 0.05, *** P < 0.001.
| Variable | Adjusted R2 | B | β | P Value | 95%CI for B | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Total | 0.056 | |||||
| Constant | 4.968 | 0.000 | 4.593 | 5.342 | ||
| CCCS | 0.127 | 0.243 | 0.000 | 0.069 | 0.184 | |
| Low back/Lower limb | 0.148 | |||||
| Constant | 4.237 | 0.000 | 3.618 | 4.855 | ||
| CCCS | 0.222 | 0.394 | 0.000 | 0.124 | 0.320 | |
| Whole body | 0.057 | |||||
| Constant | 5.698 | 0.000 | 5.000 | 6.396 | ||
| CCCS | 0.112 | 0.265 | 0.025 | 0.014 | 0.210 | |
Abbreviations: BMI, body mass index; CCCS, the Cleveland clinic constipation score.
a B, unstandardized coefficients; β, standardized coefficients.
4. Discussion
Authors previously reported that the stool consistency was associated with pain perception in healthy subjects. Specifically, the more watery their stool was, the more sensitive were the healthy subjects to painful stimuli (3). In contrast to healthy subjects, in the current study, patients with chronic pain showed that constipation was significantly and positively associated with the pain severity of the total patients and patients with low back and/or lower limb, and whole body pain. However, there were no significant associations between the stool consistency and the pain severity.
Constipation is associated with the GM composition. For example, the patients with constipation rigorously reduced abundance in Prevotella and increased representation in several genera of Firmicutes compared with the controls (8). Khalif et al. (16), reported lower amount of Lactobacillus and Bifidobacteria species in the stool sample of adults with chronic constipation. Moreover, short-chain fatty acids generated from the enteric bacterial fermentation of undigested carbohydrates may contribute to the pathophysiology of constipation (9).
The GM has an influence on autism, major depression, and Parkinson disease (17). In a study on healthy volunteers, those who took specific probiotics (Lactobacillus belveticus and Bifidobacterium longum) displayed less anxiety and depression (18). The GM contributes to the modulation of multiple neurochemical and neuro-metabolic pathways (11, 12). These pathways involve the hypothalamic-pituitary-adrenal axis, chemokines and cytokines, and autonomic nervous and enteric nervous systems, which constitute the microbiota-gut-brain axis (10). Also, brain function and psychological makeup are considered to have a reciprocal relationship with GM. Furthermore, GM can release neuroactive molecules (such as acetylcholine, catecholamine, γ-aminobutyric acid, histamine melatonin, and 5-hydroxytryptramine (5-HT) similar to the host that may induce neuropeptide production in the brain, and increase gut-blood barrier and blood-brain barrier (BBB) permeability (19, 20). The 5-HT plays an important role in the regulation of peristalsis (21), pain perception (21, 22), mood, and cognition (23). Despite its well-known role in the central nervous system, while only 5% out of the whole human body 5-HT is found in the brain, the gut contains 95% of 5-HT (24). Since 5-HT is synthesized from essential amino acid tryptophan, the increasing microbiota deterioration reduces the functionality of the tryptophan absorption in the gut, thereby reducing 5-HT biosynthesis (25).
The endogenous pain modulatory mechanisms, involving both opioid and 5-HT signaling, are impaired in patients with chronic pain (26). The dysfunction of endogenous pain modulatory mechanisms is observed in patients with whole body pain (e g, widespread pain, fibromyalgia) rather than local pain (27). Previous research suggested that patients with fibromyalgia reduced 5-HT levels, and reduced tryptophan absorption (25). Low tryptophan absorption induces low 5-HT synthesis that causes fibromyalgia symptoms (25). The current study results indicated an association between constipation and pain severity in patients with chronic pain, especially in patients with whole body, low back and/or lower limb pain. Based on the current study results, it was thus postulated that dysbiosis might have disrupted pain-modulation systems, thereby leading to a vicious cycle in which biological factors could have aggravated the pain intensity of patients with low back and/or lower limb, and whole body pain.
The constipation was associated with insufficient physical activity and excessive sedentary behavior. The mild to moderate physical activity showed positive effects on constipation (28, 29). Also, it is well known that inactivity is a risk factor for development of chronic pain (30). Moreover, increase in physical activity attenuates the severity of symptoms in patients with chronic pain (31). One of the mechanisms by which the exercise induced hypoalgesia is thought to involve the endogenous pain modulatory system (32). Additionally, it is reported that the regular exercise influences the composition and function of human GM (33). Therefore, it is suspected that the physical activity, GM, and the endogenous pain modulatory function are correlated with patients with chronic pain.
The stool consistency and constipation may be affected by age, gender, and BMI (29, 34), but CCCS had no correlation with these factors. Although the BSFS was correlated with age and BMI, the correlation coefficients were small (rs = -0.116, -0.174). In addition, BSFS and CCCS did not show gender differences. Thus, it was thought that stool consistency and constipation had little influence on age, gender, and degree of obesity.
The pain severity was correlated with CCCS, but was not correlated with BSFS. There may be a relationship with CCCS and BSFS, since they had negative correlation. However, the correlation coefficient was small. Constipation does not necessary mean a hard stool. Furthermore, BSFS is a graded scale from 1 to 7. Therefore, it was thought that BSFS did not have a significant association with the pain severity. On the other hand, authors previously reported that BSFS was associated with the pain perception in healthy subjects (3). One of the reasons might be that the subjects of the authors’ previous study were younger than the subjects of the current study. Another reason might be that although BSFS was associated with pain perception in healthy subjects, the pain perception was induced by painful external stimuli. Therefore, further studies are necessary to investigate the difference between healthy subjects and patients with chronic pain.
Medication has several side effects, especially gastrointestinal effect. Although opioid, pregabalin, and antidepressant out of the drugs listed in the current study are known to cause constipation (35-38), there were some differences of the prescribed ratio only in acetaminophen. Furthermore, there were no significant differences in BSFS and CCCS among medications. It was thus postulated that medication would hardly have influenced the current study findings.
There were several limitations to the current study due to the inclusion of elements of a qualitative study. First, the study did not measure GM composition and richness, and blood levels of substances such as short-chain fatty acids. There is growing evidence that microbiota diversity can change variations in short-chain fatty acids (39). Secondly, authors’ previous study showed that stool form consistency was associated with pain perception (3), which was not consistent with the current study results. Authors’ previous study was conducted on young healthy subjects and, in contrast, the current study was conducted on older patients with chronic pain; therefore, these results could be inconsistent. Further studies should evaluate the relationship between GM and pain perception in older adults and patients with chronic pain using 16S rRNA analysis or by measuring short-chain fatty acids. Thirdly, the intensity of pain was affected by the dosage of the prescribed medications. Fourthly, the patients were classified into five groups based on the anatomical part of the body in which the patients felt pain. Even if the part with pain was the same, it included various diseases. Further studies are needed to investigate the influence of the dosage of the prescribed medications and the underlying disease. Finally, the current study did not evaluate the effects of endogenous pain modulatory molecules including the 5-HT.
4.1. Conclusions
The results of the current study showed that constipation was significantly and positively associated with the pain severity in the total patients and patients with low back and/or lower limb, and whole body pain.