1. Background
Laparoscopic nephrectomy has become the preferred technique for living kidney donation since it has several advantages such as a smaller incision and a shorter recovery period, as compared to open surgery. Despite the reduced postoperative pain in the laparoscopic surgical technique, the patient still experiences a significant pain in the immediate postoperative period. Effective postoperative pain management is one of the keys to the early recovery of the donor (1).
Patient-controlled opioid analgesia, epidural analgesia, or a combination of both is the preferred pain management following a laparoscopic abdominal surgery. Opioid infusion with intravenous (IV) boluses or with a patient-controlled analgesia (PCA) system appears to be unreliable to provide adequate pain management after a major abdominal surgery and it has serious side effects such as respiratory depression, sedation, pruritus, nausea, and vomiting. A combination with regional analgesia can reduce the perioperative opioid consumption and associated side effects. Epidural analgesia is performed by injecting local anesthetic with or without adjuvant drugs into the epidural space with or without a catheter inserted in the epidural cavity. Epidural analgesia has an excellent analgesia profile compared to solely IV opioid analgesia, and a continuous administration provides persistent analgesia in comparison with the intermittent boluses. The transversus abdominis plane (TAP) block has been increasingly used as a regional technique for postoperative pain management in the abdominal surgery. The subcostal TAP block involves intercostal nerves innervation and produces the blockade on the upper quadrant up to T6 - T9. The posterior injection of TAP block involves the anterior-lateral cutaneous branches that pass through TAP and possibly paravertebral spread to produce spinal afferent nerves blockade and control the somatic pain on the lower quadrant from T7 to L1 (2).
Being neuraxial analgesia in nature, epidural analgesia has been associated with post-dural puncture headache, hypotension, postoperative urinary retention, and delayed mobilization of the donor. These side effects open up the possibility of using another regional analgesia technique such as the TAP block. A previous study suggested additional dexamethasone to prolong the analgesic effect of the TAP block up to 24 hours after a cesarean section (3-5). Our study investigated the effect of three-quadrant ultrasound (US)-guided bilateral posterior and unilateral subcostal TAP block using 0.25% bupivacaine with additional dexamethasone 8 mg to prolong the analgesia effect in comparison with the continuous epidural analgesia using 0.125% bupivacaine for the pain management following transperitoneal laparoscopic living donor nephrectomy (6, 7). The primary outcome was cumulative morphine consumption in the first 24 hours after surgery. The secondary outcomes were the numerical rating scale (NRS), the first-time mobilization, and duration of urinary catheterization postoperatively.
2. Methods
This research was a prospective, randomized controlled clinical study, involving 50 adult patients with American Society of Anesthesiologists (ASA) classification I - II who underwent transperitoneal laparoscopic donor nephrectomy with a Pfannenstiel incision for kidney extraction. The research protocol was approved by the Research Ethics Committee of Universitas Indonesia (number 17-05-0432) and registered at ClinicalTrial.gov (NCT03154436). Exclusion criteria were body mass index (BMI) > 30, age < 18 or > 65 years old, the chronic use of analgesics or anti-inflammatory drugs, neuropathy, and allergy to local anesthetics. After obtaining the written informed consent, the patients were randomly allocated to either a TAP block group or a continuous epidural group using a computer-generated randomization sequence (http://www.randomization.com) list of random numbers, which was performed by sealed envelopes.
All patients received midazolam 2 mg IV and ranitidine 50 mg IV as premedication. Heart rate, continuous electrocardiography, non-invasive blood pressure, pulse oxygen saturation, and end-tidal carbon dioxide were monitored. Intubation was facilitated with propofol 1 - 2 mg/kg IV, fentanyl 2 µg/kg IV, and atracurium 0.5 mg/kg IV. The general anesthesia was maintained using sevoflurane 1.5 - 2% and atracurium 0.005 mg/kg/min. Fentanyl boluses 1 µg/kg IV were given when necessary for intraoperative pain relief during the surgical stimulations. Two anesthetist consultants performed all the blocks. For the epidural group, the epidural catheter was placed early before anesthesia induction. The patient was prepared in a sitting position and after aseptic preparation of the epidural location, a Perifix® (BBraun, Germany) Tuohy needle was inserted between T12 and L1, and then the catheter was implanted 6 cm inside the epidural space. The vacuum catheter aspiration followed by the negative test dose using 60 mg of 2% lidocaine with 15-µg epinephrine produced no changes in extremity motoric sensation, heart rate, and blood pressure, confirming the correct catheter location. After the completion of the surgery, the initial bolus 3 ml followed by 6 mL/hour of 0.125% bupivacaine started.
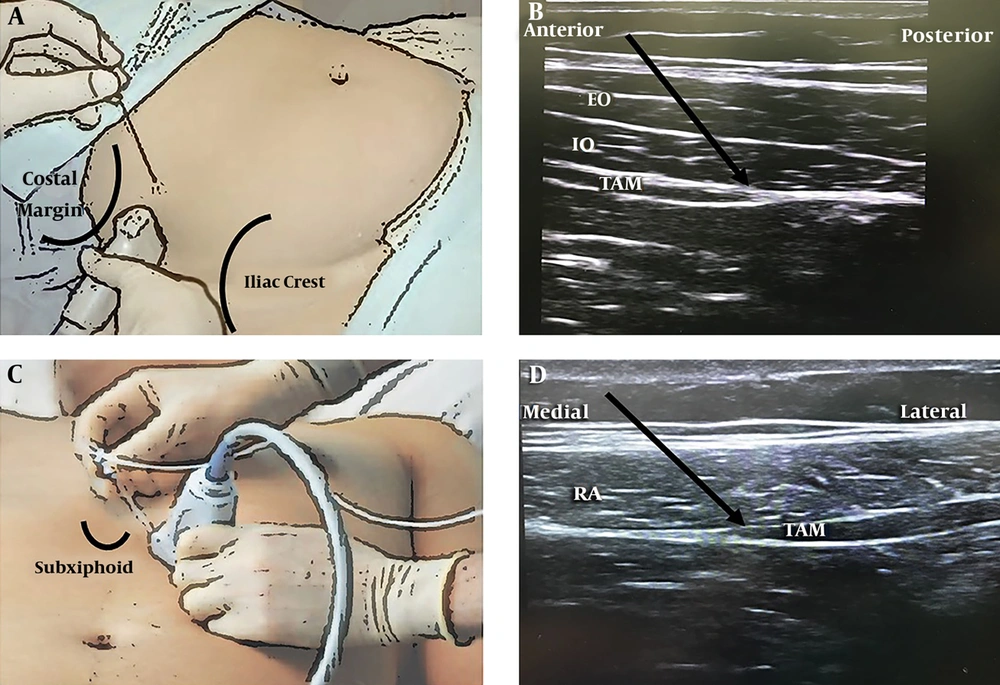
The TAP block group patients received the block after the completion of the surgery before gaining the consciousness from the general anesthesia. The patients were in a supine position to receive the bilateral posterior TAP block injection. Then, the unilateral subcostal TAP block injection was performed on the side of the retrieval kidney. For the posterior TAP injection, a linear transducer (L6-12 Mhz, Logic P7, GE Healthcare) was placed transversely posterior to the mid-axillary line at the level of the umbilicus between the iliac crest and the costal margin as identification of the lumbar “triangle of Petit”. The external oblique, internal oblique, and transversus abdominis muscle fascia that then became aponeurosis were visualized. A Stimuplex® (BBraun, Germany) 20G 100-mm needle was advanced in an anteroposterior direction between the transversus abdominis and internal oblique muscle layer, superficial to the transversus abdominis aponeurosis. For the subcostal TAP injection, the linear transducer was placed inferior to the xiphoid process and parallel to the costal margin. The needle was advanced to the TAP and located below the rectus abdominis muscle, the aponeurosis of the linea semilunaris, between the internal oblique muscle and transversus muscles. The 2 mL of 0.9% saline was first injected to verify the correct position of the needle; then, 20 mL of 0.25% bupivacaine plus dexamethasone 8 mg was deposited at each of three-quadrant TAP block injection and seen as a dark elongated shape of the local anesthetic spreading in the TAP. The total amount of bupivacaine in the TAP block group was 150 mg for each patient (Figure 1).
The TAP block technique, posterior injection (A), an ultrasound image of the posterior approach (B), subcostal injection (C), an ultrasound image of the subcostal approach (D). EO, external oblique muscle; IO, internal oblique muscle; RA, rectus abdominis muscle; TAM, transversus abdominis muscle.
At the end of the surgery, all patients received a combination of Prostigmin 0.02 - 0.04 mg/kg IV and atropine 0.02 mg/kg IV to reverse neuromuscular blockade, as well as omeprazole 20 mg IV and ondansetron 4 mg IV to treat postoperative nausea and vomiting (PONV), followed by extubation. Both study groups received patient-controlled analgesia (PCA) morphine using a PCA Infusion Pump (Perfusor®, BBraun, Germany), with the setting of initial bolus loading dose 1 mg, demand dose 1 mg, lockout time 10 minutes, maximum dosage 6 mg, and without continuous basal infusion. The NRS (none = 0 to worst pain = 10) at rest, during movement (by coughing), and morphine consumption was recorded at 2, 6, 12, and 24 hours after the surgery. The patient’s first-time mobilization was defined as the first time ability to sit on the bed with minimum assistance. The duration of urinary catheterization depended on the surgeon assessment.
An independent assistant recorded the data. The primary investigator was blinded to the entire data recording. Statistical analysis was performed using IBM SPSS version 21.0. Patient characteristics were presented in a tabular form to assess data distribution. Numerical data were tested with unpaired t-test or Mann-Whitney U test. Categorical data were tested by the Chi-square test. Data were presented as means (± standard deviation) or median (minimum - maximum). The sample size calculation was based on the assumption of a 20% difference in the postoperative opioid consumption in the first 24 hours between the groups. A sample size of 23 in each group was estimated to detect significant differences (P < 0.05) with the power of 80%, and 50 patients were recruited to compensate for dropouts.
3. Results
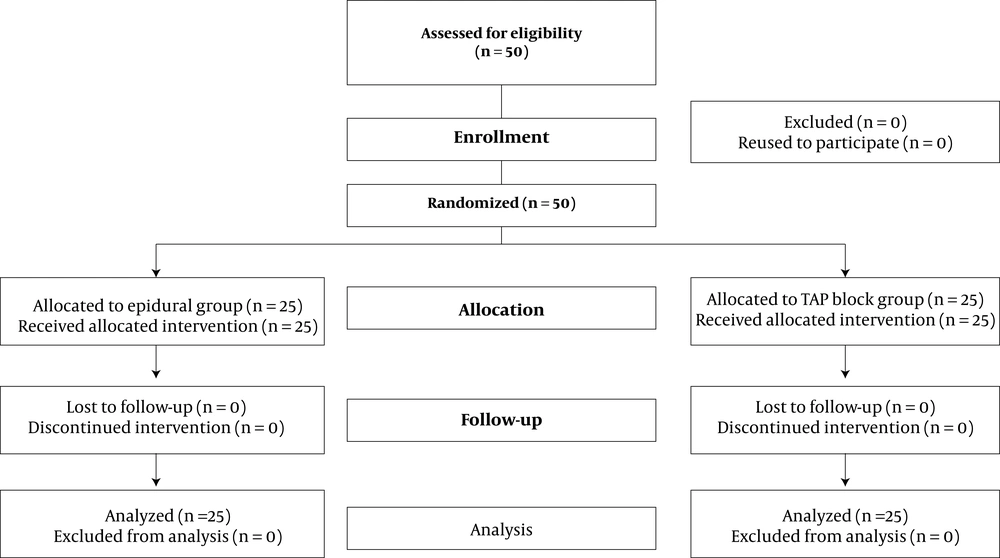
50 patients were enrolled, randomly allocated to a TAP block group (25 patients) or an epidural group (25 patients), and analyzed (Figure 2). The subject’s characteristics and intraoperative fentanyl consumption were not significantly different between the groups (Table 1).
| Characteristic | TAP Block (n = 25) | Continuous Epidural (n = 25) |
|---|---|---|
| Sex | ||
| Male (%) | 18 (72) | 18 (72) |
| Female (%) | 7 (28) | 7 (28) |
| Age (y) | 31 (25 - 54) | 38 (23 - 58) |
| Weight (kg) | 65.34 ± 9.21 | 65.15 ± 9.76 |
| Height (cm) | 165.78 ± 7.82 | 162.38 ± 5.82 |
| Body mass index (BMI) | 24.46 ± 3.27 | 24.92 ± 3.27 |
| ASA | ||
| I | 18 (72%) | 12 (48%) |
| II | 7 (28%) | 13 (52%) |
| Intraoperative fentanyl (µg) | 400 (300 - 850) | 400 (300 - 550) |
| Blood glucose | ||
| Postoperative baseline | 128 (113.74 - 145.54) | 134 (124.57 - 145.41) |
| Postoperative 24 hours | 120 (100 - 203) | 105 (96 - 124) |
aCategorical variable presented in No. (%), Numeric variable presented as means ± standard deviation or median (minimum - maximum).
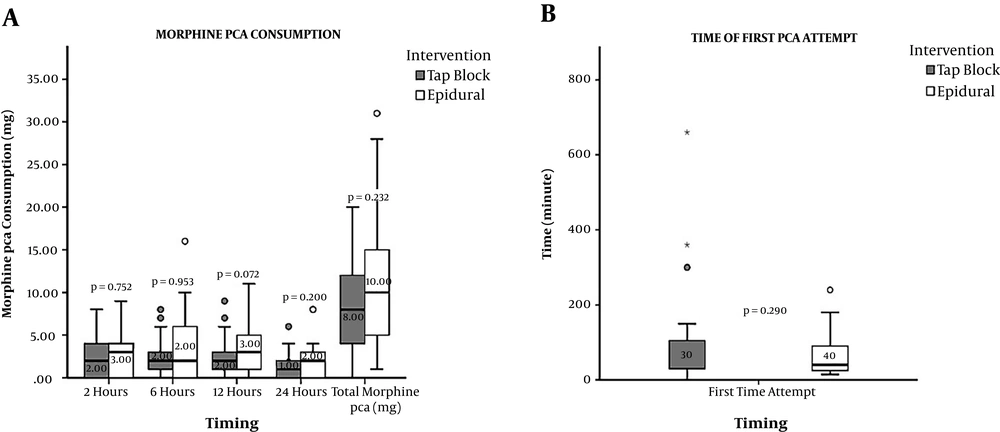
The highest morphine consumption occurred at the time of 12 hours after surgery with a median value of 2.0 (0 - 9) mg in the TAP block group while it occurred 6 hours after surgery with a median value of 3.0 (0 - 16) mg in the epidural group. The median values of morphine consumption at 2, 6, 12, and 24 hours were not significantly different between the TAP block and continuous epidural block (P = 0.752, P = 0.953, P = 0.072, and P = 0.200). The total cumulative morphine consumption in the first 24 hours (P = 0.232) and the first-time attempt of PCA were not significantly different between the groups (Figure 3).
Comparison of postoperative morphine consumption and the time of first PCA attempt. Gray box represents the TAP group and white box represents the epidural group. The horizontal lines indicate the medians, boxes indicate interquartile ranges, and whiskers indicate ranges. The P values are presented in median (minimum - maximum), P < 0.05 is significant. PCA, patient-controlled analgesia. NRS, numerical rating scale.
Table 2 shows that the median NRS value at rest at any time point and the mean NRS in the first 24 hours were not significantly different between the TAP block and continuous epidural block. The median NRS value during movement at any time point and the mean NRS in the first 24 hours were not significantly different between TAP block and continuous epidural block, except at the time of 12 hours (P = 0.004). The first-time mobilization after surgery was similar in the groups. The duration of urinary catheter usage after surgery was significantly longer in the continuous epidural group than in the TAP block group.
| TAP Block (n = 25) | Continuous Epidural (n = 25) | P Value | |
|---|---|---|---|
| NRS at rest | |||
| 2 hours | 1.00 (0 - 3.00) | 1.00 (0 - 3.00) | 0.716 |
| 6 hours | 1.00 (0 - 3.00) | 1.00 (0 - 3.00) | 0.637 |
| 12 hours | 1.00 (0 - 3.00) | 1.00 (0 - 4.00) | 0.066 |
| 24 hours | 1.00 (0 - 3.00) | 1.00 (0 - 3.00) | 0.587 |
| Mean in the first 24 hours | 1.83 ± 1.15 | 2.33 ± 1.55 | 0.207 |
| NRS in movement | |||
| 2 hours | 3.00 (0 - 6.00) | 3.00 (0 - 5.00) | 0.713 |
| 6 hours | 3.00 (1.00 - 5.00) | 3.00 (0 - 6.00) | 0.696 |
| 12 hours | 2.00 (0 - 5.00) | 3.00 (0 - 6.00) | 0.004b |
| 24 hours | 2.00 (0 - 5.00) | 3.00 (1.00 - 6.00) | 0.213 |
| Mean in the first 24 hours | 5.31 ± 1.71 | 6.08 ± 2.20 | 0.172 |
| Mobilization | |||
| First-time mobilization (hours) | 7.00 (1.00 - 22.00) | 10.00 (2.00 - 24.00) | 0.075 |
| Duration of urinary catheter use (hours) | 28.00 (20.00 - 46.00) | 44.00 (26.00 - 48.00) | < 0.001b |
aMann-Whitney test for non-parametric data, presented as median (minimum - maximum). Unpaired independent t-test for parametric data, presented as means + standard deviation. NRS and morphine consumption at 0 hours were constantly 0.
bP < 0.05 is significant.
4. Discussion
Effective postoperative analgesia provides an adequate comfort level along with acceptable side effects and produces early ambulation after surgery. The multi-modal analgesic regimen includes non-opioid analgesics such as local anesthetic to provide better postoperative pain relief and reduce side effects of opioids (8). An optimal analgesic technique suppresses all the noxious stimuli involving parietal and visceral components from a surgical injury that engages peripheral and central sensitization from the primary afferent nociceptors and spinal dorsal horn neurons. Epidural analgesia is a commonly practiced regional neuraxial analgesia technique for abdominal surgery that covers both parietal and visceral components (9). Epidural continuous approach techniques are associated with better pain relief compared to single injection nerve blocks. However, despite a reliable pain relief, it has several side effects such as paresthesia, hypotension, urinary disturbance, and complications such as inadvertent intra-thecal migration of the epidural catheter or epidural hematoma (10). With an increase in the number of living kidney donations, it is important to optimize comfort and reliable but safe postoperative analgesia for the donor.
The ultrasound-guided TAP block has increasingly performed with lower complications as an interfascial plane block for abdominal surgery analgesia (11). Our study demonstrated that the effects of three-quadrant TAP block using 0.25% bupivacaine with additional dexamethasone 8 mg and continuous epidural block using 0.125% bupivacaine were not significantly different in postoperative morphine consumption and pain scale 24 hours after transperitoneal laparoscopic donor nephrectomy. A study on patients undergoing hand-assisted laparoscopic nephrectomy showed that TAP block reduced pain scores and decreased total morphine consumption in the first 24 hours (1). However, a meta-analysis showed that TAP block as part of multimodal analgesic regimen was superior to a placebo block or IV analgesics alone in reducing analgesic consumption and pain level only during 2 - 6 hours after laparoscopic abdominal surgery (6) We chose the bilateral posterior and unilateral subcostal approach using 0.25% bupivacaine to provide adequate analgesia for the lower quadrant and the upper-half quadrant of the abdomen. The ultrasound-guided posterior TAP block produced a longer analgesia with higher patient satisfaction and lower mean values of pain score after cesarean section compared to the lateral TAP block (11). The local anesthetic in the posterior approach provided somatic pain relief by blocking the lateral cutaneous branches of thoracolumbar nerve before branching and spreading regionally in the neurofascial TAP. It can reach into the paravertebral space to block the thoracolumbar sympathetic system from T7 to L1 (2). We used a combination of bilateral posterior and unilateral subcostal approach TAP block with additional dexamethasone 8 mg in each injection to prolong the duration of analgesia. Additional glucocorticoid dexamethasone as a new adjunct to local anesthetics can be utilized to improve the duration of regional analgesia. Recent studies have shown that ultrasound-guided bilateral TAP block at the end of the cesarean section using 40 mL ropivacaine 0.2% and dexamethasone 8 mg prolonged the duration of TAP block and in patients undergoing open abdominal hysterectomy, the additional dexamethasone 8 mg to 0.25% bupivacaine improved the quality and duration of bilateral TAP block (7). The mechanism involved in the prolongation of analgesia was suggested to be the systemic effect of dexamethasone on the inhibition of nociceptive C-fibres, as well as the anti-inflammatory effects. A meta-analysis by Choi et al. showed that the use of perineural dexamethasone significantly prolonged the duration of analgesia approximately by 6 hours compared to the use of IV dexamethasone. A recent meta-analysis by Hussein et al. showed that irrespective of dose, the use of perineural dexamethasone does not appear to provide a significant incremental benefit to the pain score, duration of analgesia or motor blockade, and cumulative opioid consumption when compared to the use of IV dexamethasone at a 24-hour follow-up (12).
Our study showed blood glucose after the surgery was non-significantly higher in the TAP block group due to additional dexamethasone; however, no symptom or complication due to hyperglycemia was observed. Our result was similar to the findings of a previous study that showed a non-significant difference in blood glucose level between additional dexamethasone and placebo groups (13). This suggests adding dexamethasone cautiously in diabetic patients although this hypothesis must be confirmed by further research in this patient population. Williams et al. evaluated the toxicity of perineural adjuvants to ropivacaine in an animal model and proved the administration of supratherapeutic doses of dexamethasone for two hours did not appear to cause neuronal cell death (12). Future studies should focus on evaluating the dose-dependent analgesic effects of perineural or interfascial dexamethasone addition.
The total dose of bupivacaine used was 150 mg in the TAP block and less than 180 mg in the continuous epidural group. The efficacy of bupivacaine with different concentrations has been studied in peripheral nerve blocks. Research findings suggested that patients receiving 0.25% bupivacaine had lower pain with higher satisfaction while the duration of anesthesia showed no difference compared to the group receiving 0.5% bupivacaine (14). We chose 0.25% bupivacaine for the TAP block since those concentrations produce good analgesia and prevent local anesthetic toxicity. The concentration of 0.125% bupivacaine for the continuous epidural block was proven effective for postoperative pain management after an abdominal surgery with a lower incidence of leg weakness and paresthesia (15). In our study, the TAP block group had a lower NRS during movement, especially at 12 hours after surgery (P = 0.004). The epidural was attached to the T12 - L1 level and the administration of 3 mL bupivacaine 0.125% as the initial bolus followed by 6 mL per hour might only reach the T10 - L1 level. The main target was postoperative pain from the Pfannenstiel incision, requiring analgesia at the T11 - L1 level that could be resolved by bilateral TAP blocks and continuous epidurals in this study. In the epidural group, trocar incision in the upper abdomen may not be blocked, which could cause pain, especially when the patient was moving. However, if the epidural block was performed at a higher level, it would be necessary to use a higher dose of bupivacaine with a larger volume to be able to block approximately from T7 - T8 to L1.
TAP block and opioids can control the parietal pain, whereas steroid controls the visceral pain (10). Our primary outcome was opioid consumption during the first 24 hours after surgery. The PCA device was used to administer morphine under patient control without a continuous basal infusion in our study. Intravenous PCA enhances morphine delivery when the parenteral route is needed for postoperative systemic analgesia based on patients demand who self-administered the drug only when they felt pain (8, 16). We avoid the basal infusion of morphine as recommended since our subjects were all opioid-naive patients (17). The morphine consumption at all measurement times and the cumulative consumption in the first 24 hours after surgery were not significantly different between the TAP group and the continuous epidural group. This result was similar to another study finding that showed ultrasound-guided TAP block following hysterectomy did not significantly decrease the postoperative fentanyl consumption and pain score compared to standard treatment (18). The first-time mobilization after surgery was not significantly different between the groups. Although the surgeon removed the urinary catheter as soon as the patient no longer needed it, the duration of urinary catheterization was significantly longer in the epidural group as a complication that prevented patients from early returning to the normal function.
This study faced several limitations. The sensory testing to evaluate the block level was not recorded. This study did not assess nausea, vomiting, and epigastric pain because the antiemetic was given immediately to prevent those complications owing to the laparoscopy procedure. However, relieving those gastrointestinal discomforts could reduce bias while assessing the postoperative pain in this study. This is an open-label study in which the epidural catheter insertion was done before surgery with a small test dose bolus; therefore, its’ effects might be subsided at the end of the surgery. The epidural block started with a bolus followed by a continuous rate infusion after surgery, which was similar to the TAP block but there was still a bias. We excluded patients with BMI > 30; thus, the results of this study cannot be generalized to that population. For the future study, it is better to compare the TAP block with/without dexamethasone and the epidural block.
4.1. Conclusion
The present study showed the analgesic effect of three-quadrant TAP block with additional dexamethasone was not significantly different from the effect of continuous epidural analgesia for the first 24 hours after surgery. Therefore, it can be an alternative approach as part of multimodal analgesia following transperitoneal laparoscopic donor nephrectomy and other lower abdominal surgeries with Pfannenstiel incision.