1. Background
Colonoscopy is a standard method to diagnose, screen, treat, and follow up many diseases related to rectal, colon and part of the terminal ileum (1-3). Some patients can tolerate colonoscopy without sedation and analgesia, but the procedure is painful for most patients. Most gastroenterologists use intravenous sedation for all procedures except for flexible sigmoidoscopy (4-6). The most commonly used approach to endoscopic sedation is intravenous conscious sedation by administering benzodiazepines alone or in combination with narcotic drugs. In a systematic review of 36 studies, sedation procedure was proven to have a high level of patients’ and doctors’ satisfaction, with a low level of serious complications (7-9).
Midazolam is prescribed for endoscopic sedation. Midazolam has a sedative, anti-anxiety, amnesic effect, but it is not an analgesic (1, 5). Narcotic drugs such as meperidine and fentanyl are commonly used for endoscopy. They should be used with benzodiazepine carefully and at low doses for elderly patients, those with severe liver and kidney diseases, and those who have a history of seizure. Since the onset and clearance of fentanyl are rapid, it is preferred over meperidine (1, 5).
The time for the onset of fentanyl effect is 2 - 3 minutes and the duration of its effect is 30 to 60 minutes with no amnesia effect. It is given intravenously at a dose of 0.5 - 1 mcg/kg. Its most important complication is respiratory depression, which is exacerbated by the simultaneous administration of sedation, and its most serious complication is cardiovascular complications (low blood pressure and heart rate). Risk factors for these complications include old age, underlying diseases, especially pulmonary diseases, dementia, and anemia (1, 5).
Propofol is the most common intravenous anesthetic drug that has clinical use. The onset and the end of its effect are fast. The onset of hypnosis after a dose of 2.5 mg/kg is fast. The most prominent cardiovascular complication of propofol is the reduction of blood pressure (1, 5, 10). If fentanyl and propofol are used simultaneously, side effects are exacerbated; so, the risk of apnea and hemodynamic changes will be higher and can challenge the anesthetist (1, 5).
Dexmedetomidine is a well-known sedative drug that has sedative, analgesic, and sympatholytic effects by activating adrenergic alpha 2 receptors in the brain and spinal cord. Its analgesic effects are due to the agonist effects of the alpha 2 receptor on the posterior horn of the spinal cord (1, 2, 9).
The half-life of dexmedetomidine is two to three hours. Unlike in the case of other sedatives, patients, sedated with dexmedetomidine, will go back to the previous level of consciousness by stimulation. Dexmedetomidine causes less respiratory depression than other sedatives and does not cause respiratory depression even at high doses. Additionally, it does not have additive effects in combination with propofol and does not exacerbate respiratory depression caused by propofol (2, 3, 10-13). Dexmedetomidine reduces MAP (mean arterial pressure) (13%) and heart rate (29%). It only may cause severe bradycardia in a small percentage of patients (heart rate of less than 40 per minute), which responds with an anticholinergic, and there is no risk for rebound even after prolonged (24 hours) infusion (10).
Due to the possibility of rare but severe cardiovascular complications of sedative medications, there is a need for monitoring the blood pressure, heart rate, pulse oximetry and in some cases, electrocardiography during colonoscopy (14). The lack of stimulation induced by the procedure and the presence of active metabolites of the drug in the circulation can increase the risk of respiratory suppression during the post-procedure period (1, 5).
One of the important challenges for doctors in colonoscopy is the complications of anesthetic drugs, hemodynamic changes, and respiratory depression. These facts have led researchers to always seek and test newer drugs to find an alternative with lower risks.
Considering the complications of fentanyl, such as respiratory depression and the risk of apnea, and the potential of being abused by the medical staff and its additive effects in combination with propofol on hemodynamic changes and respiratory depression, and given the beneficial effects and reduced complications of dexmedetomidine (lower respiratory depression), and considering that there are a few studies on the examination of dexmedetomidine in general anesthesia and colonoscopy, without any randomized clinical trial study of dexmedetomidine and fentanyl on analgesia during colonoscopy, we decided to compare the analgesic effects and hemodynamic changes due to dexmedetomidine and fentanyl during colonoscopy.
2. Objectives
The aim of this study was to compare the analgesic effect and hemodynamic changes due to dexmedetomidine and fentanyl during elective colonoscopy.
3. Methods
This randomized clinical trial was performed on 80 American Society of Anesthesiologists (ASA) class I or II patients who underwent elective colonoscopy at Rouhani Hospital from November 2016 to August 2017. The inclusion criteria were an age from 20 to 70 years, ASA class I or II, and elective colonoscopy. The exclusion criteria included an age of less than 20 or more than 70 years, ASA class ≥ 3, cardiovascular diseases (arrhythmia, aortic stenosis, Ischemic heart disease, hypertension, heart failure (ejection fraction < 30%), liver diseases (Child-Pugh classification C), kidney diseases, lack of patient cooperation, and psychiatric disorders (major depression, mania, psychosis, drug addiction, systolic blood pressure < 90 mmHg, and heart rate (HR) < 50/min). The research subject and methodology were explained to the patient’s companions and the process was performed after taking their written consent. This study was also approved by the Ethics Committee of the Babol University of Medical Sciences with No. 2306 and registered at the Iranian Registry of Clinical Trials under the code of IRCT201602297752N6.
The size of the sample assuming the mean pain score difference of 1.5 units, standard deviation of 2.5, and a power of 80% with a confidence interval of 95%, was obtained to be 30 in each group while 40 subjects were included in each group to raise the test power.
The syringes were coded by an anesthetic nurse who was not involved in the process of sedation and patient evaluation. Syringes containing drugs were selected similarly in terms of volume. The patients, anesthetists, colonoscopist, and patient assessor (anesthetist’s assistant) were blind to the drug regimen. The patients were randomly (computer-generated) divided into two groups (Figure 1).
In the intervention group (group D), dexmedetomidine (Precedex) (Huspiria of USA, Behestan Pharmaceutical CO., Iran) was prescribed at 1 mcg/kg within 10 minutes before colonoscopy, followed by 0.5 mcg/kg/hour during colonoscopy while the control group (group F) was taken fentanyl (Caspian Darou, Rasht, Iran) at 0.5 mcg/kg three minutes before starting the colonoscopy. In the fentanyl group, the normal saline infusion was used as maintenance.
The pain score based on the visual analogue scale (Figure 2), blood pressure, heart rate, respiratory rate, and SpO2 were recorded from starting the colonoscopy (time 0) and every 5 minutes until the recovery. Propofol 20 mg (Propofol- ® Lipuro 10 mg/ml, B Braun AG, Melsungen, Germany, Daroupakhsh, Tehran, Iran) was prescribed as the rescue dose if needed (pain or severe discomfort during colonoscopy) during the procedure. The duration of colonoscopy, patients’ satisfaction with colonoscopy, and colonoscopists’ satisfaction (Figure 3) were evaluated before the discharge. The frequency of respiratory depression, hypotension, and bradycardia (HR < 50/min) was recorded. Respiratory depression was treated with positive pressure ventilation with a mask, hypotension with intravenous ephedrine (10 mg), and bradycardia with intravenous (0.5 mg) atropine.
Data were analyzed using SPSS V.22 software. The comparison of the two groups was performed for blood pressure, respiratory rate, heart rate, SpO2, endoscopist’s and patient’s satisfaction, and pain score based on the t-test. The Chi-square test was used to compare the incidence. P value ≤ 0.05 was considered significant.
4. Results
From November 2016 to August 2017, a total of 90 patients were enrolled. 6 patients were excluded due to the lack of readiness and four patients were excluded for their age (Figure 1). The study was performed on 80 patients divided into two groups of 40 (group D: Dexmedetomidine and group F: Fentanyl). There was no significant difference in age, sex, education level, body mass index, and primary clinical parameters between the two groups (Table 1).
| Characteristics | D | F | P Value |
|---|---|---|---|
| Age (y) | 49.20 ± 13.98 | 48.88 ± 13.84 | 0.92 |
| Body mass index | 24.68 ± 2.72 | 23.80 ± 2.36 | 0.13 |
| Education level | 0.19 | ||
| Uneducated | 2 (2.5) | 1 (1.3) | |
| Diploma degrees | 34 (42.5) | 29 (36.3) | |
| Bachelor’s degrees and higher | 4 (5) | 10 (12.5) | |
| Sex (male) | 21 (52.5) | 24 (60) | 0.65 |
| SpO2% (pulseoximetry) | 98.55 ± 0.75 | 98.30 ± 0.27 | 0.15 |
| Respiratory rate (number/min) | 13.63 ± 1.29 | 13.90 ± 1.19 | 0.33 |
| Heart rate (number/min) | 73.30 ± 11.80 | 77.97 ± 10.51 | 0.06 |
| Mean arterial pressure (mm Hg) | 87.75 ± 14.33 | 89.28 ± 14.19 | 0.63 |
Demographic and Basic Clinical Parameters in Two Groupsa
There were no significant differences between the two groups (Table 2) regarding the time to reach the cecum, duration of colonoscopy, colonoscopist’s satisfaction, and patients’ satisfaction (P > 0.05). Nine patients in the dexmedetomidine group and 40 patients in the fentanyl group received a rescue dose of propofol (P < 0.05).
| Groups | D (N = 40) | F (N = 40) | P Value |
|---|---|---|---|
| Colonoscopy time (min) | 7.82 ± 3.13 | 7.68 ± 3.09 | 0.83 |
| Secal time (min) | 5.72 ± 2.83 | 6.05 ± 2.74 | 0.60 |
| Colonoscopist satisfaction | 8.98 ± 0.77 | 8.88 ± 0.82 | 0.58 |
| Patient satisfaction | 8.70 ± 0.85 | 8.52 ± 1.32 | 0.48 |
The Mean Rescue Dose, Patient and Colonoscopist Satisfaction, and Colonoscopy Time in Two Groups
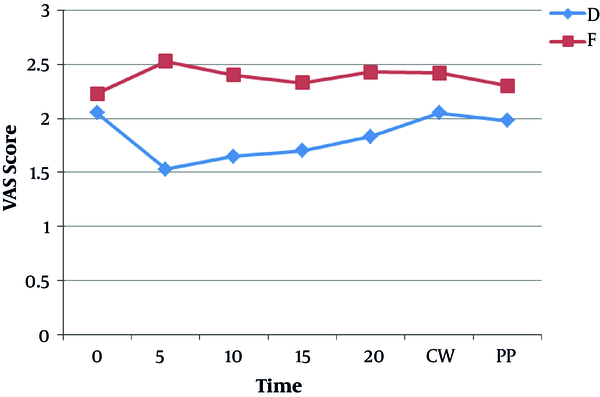
The mean and standard deviation of pain intensity (based on VAS) was lower in group D than in group F (Figure 4) and this difference was statistically significant (P < 0.05).
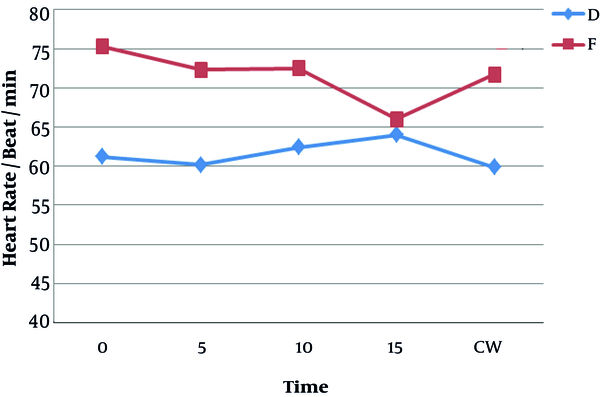
Figure 5 shows that the heart rate of patients was lower in the dexmedetomidine group than in the fentanyl group and this difference was statistically significant (P < 0.05). Severe bradycardia (heart rate of less than 40/min) was not observed in any of the patients in the fentanyl group, but one patient suffered from severe bradycardia in the dexmedetomidine group, which was solved using atropine.
Table 3 shows the mean and standard deviation of the MAP in the two groups. The MAP was not significantly different at 0, 5, and 10 minutes, as well as at CW (coming out of the colonoscopy). Nevertheless, at the 15th minute, the p-value was less than 0.05.
| Time (Min) | D | F | P Value |
|---|---|---|---|
| 0 | 77.38 ± 13.34 | 83.58 ± 14.00 | 0.046 |
| 5 | 75.50 ± 12.68 | 77.43 ± 13.67 | 0.516 |
| 10 | 75.00 ± 12.94 | 76.50 ± 12.85 | 0.698 |
| 15 | 68.67 ± 5.51 | 102.00 ± 2.83 | 0.003 |
| CW | 75.35 ± 12.48 | 76.30 ± 13.08 | 0.741 |
Mean and Standard Deviation of Mean Arterial Pressure (MAP) in Both Groups
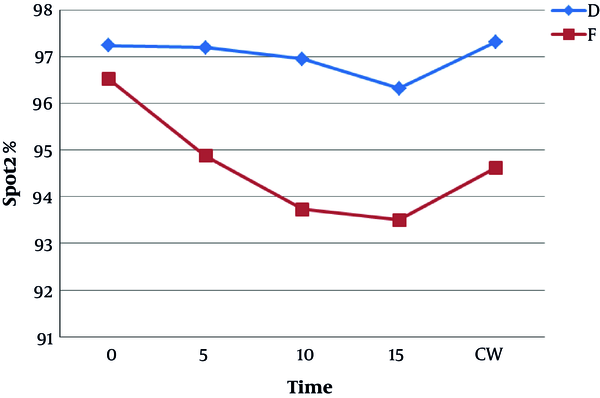
Figure 6 shows the mean and standard deviation of SpO2 in the study patients. SpO2 was higher at every minute for the dexmedetomidine group and the difference between the two groups was significant (P < 0.05).
The mean and standard deviation of respiratory rate (Table 4) at 0, 5, 10, 15 minutes, and CW (coming out of the colonoscopy) were not significantly different between the two groups.
| Time (Min) | D | F | P Value |
|---|---|---|---|
| 0 | 12.90 ± 1.43 | 13.03 ± 1.25 | 0.678 |
| 5 | 13.20 ± 1.31 | 13.15 ± 1.39 | 0.869 |
| 10 | 13.04 ± 1.11 | 13.18 ± 1.30 | 0.720 |
| 15 | 14.00 ± 1.73 | 14.00 ± 0.00 | 1 |
| CW | 13.15 ± 1.16 | 13.13 ± 1.32 | 0.929 |
The Mean and Standard Deviation of Respiratory Rate in Both Groups
5. Discussion
This study was performed on 80 patients in two equal groups with the aim of comparing the analgesic effects and hemodynamic changes due to dexmedetomidine and fentanyl during colonoscopy in patients who were candidates for elective colonoscopy.
The results of the present study showed that the pain score was significantly lower in the dexmedetomidine group than in the fentanyl group.
The study of Wu et al. was conducted on 60 patients in two equal groups to compare dexmedetomidine and midazolam in endoscopy. Variables included were peripheral oxygen saturation, heart rate, mean arterial pressure, and pain intensity based on the numerical rating scale before and after the procedure. The level of patient satisfaction was also evaluated. Of course, Fentanyl was added to both groups along with the main drug. The results showed that in the dexmedetomidine group, there was a lower pain score and higher peripheral oxygen saturation (SpO2). They concluded that dexmedetomidine is safe and effective (15).
Although the method was slightly different from the method of the present study, the results were similar. In the present study, nine patients in the dexmedetomidine group and 40 patients in the fentanyl group received rescue doses of propofol. The main reason for the administration of a higher dose of propofol in the fentanyl group was a feeling of discomfort during colonoscopy. The higher dose of propofol combined with fentanyl resulted in a higher prevalence of bradypnea and decreased peripheral oxygen saturation in the fentanyl group. Dexmedetomidine had no respiratory depression effects.
The study by Kaygusuz et al. conducted on 24 patients comparing the effect of dexmedetomidine and propofol in colonoscopy concluded that the combination of dexmedetomidine and low-dose fentanyl and midazolam could be effective and it is a good alternative for propofol. There were 24 patients in each group (16).
Due to the low pain intensity in colonoscopy and considering the sedative and analgesic effects of dexmedetomidine and the risk of bradycardia, adding fentanyl can increase the risk of bradycardia.
The study by Jalowiecki et al. was conducted on 64 patients to assess the potential analgesic and sedative effects and limitations of using dexmedetomidine in colonoscopy. Patients were divided into three groups. Group D received doses of dexmedetomidine 1 mcg/kg in 15 minutes and then, 0.2 mcg/kg/h in infusion. Group P received meperidine (1 mg/kg) and midazolam (0.05 mg/kg) and group F received fentanyl (1 - 2 mcg/kg) intravenously. The evaluated variables included heart rate, blood pressure, oxygen saturation, respiratory rate, sedation and analgesia quality, and duration of recovery to discharge. According to the plan, the study was conducted in three groups of 90 patients but the intervention stopped due to severe bradycardia (less than 40 per minute) and hypotension (less than 50% of baseline) in two patients in group D. 47% of the patients in group D, 79.2% in group P, and 42.8% in group F needed a supplement injection of fentanyl (17). Although the procedure and the evaluated variables were similar to those of the present study, the adjuvant in our study was propofol. Adding fentanyl to dexmedetomidine has no respiratory complications, but it exacerbates bradycardia and hypotension.
Dere et al. compared the hemodynamic, respiratory, and analgesic effects of dexmedetomidine at 1 mcg/kg in infusion 10 min before colonoscopy and midazolam at 0.05 mg/kg on 60 patients. The fentanyl 1 mcg/kg was added to both groups before colonoscopy. The evaluated variables included heart rate, mean blood pressure, SpO2, respiratory rate, pain intensity, and patient satisfaction. SpO2 and heart rate were higher in the midazolam group than in the dexmedetomidine group. The mean blood pressure and pain intensity were not different between both groups. The patient’s satisfaction was lower in the dexmedetomidine group (18). The findings of this study were not consistent with our findings because both groups received fentanyl. In our study, patient satisfaction was similar in the two groups, which could be due to the addition of propofol to drugs.
Another study in 2014 examined the effect of dexmedetomidine on colonoscopy in elderly people. In this study, 50 patients aged 60 - 70 years with ASA class 1 - 4 were randomly divided into two groups: Dexmedetomidine (n = 25) and midazolam (n = 25). Mepredidin 0.5 mg/kg was prescribed after initial sedation and before starting the procedure and 0.25 mg/kg was repeated if required. Hypotension and bradycardia were higher in the dexmedetomidine group, but the pain score was lower and the total required dose of meperidine significantly reduced in the dexmedetomidine group (19). Although the results were similar to our findings in terms of the analgesia and incidence of hypotension and bradycardia in the group of dexmedetomidine, unlike our study, meperidine was used instead of fentanyl.
In a study conducted by Techanivate comparing the effect of dexmedetomidine and propofol on hemodynamic parameters in colonoscopy of 70 patients, the incidence of hypotension was higher in the propofol and fentanyl group than in the combined dexmedetomidine and propofol group. This result was not consistent with our finding, which can be due to the difference in methodology. They used dexmedetomidine 1 mcg/kg for 5 minutes before starting colonoscopy in group D plus 20 mg propofol, and 0.5 mg/kg, fentanyl plus 1 mg/kg propofol in group P, but in our study, 20 mg of propofol (instead of 1 mg/kg propofol) was prescribed for group P, which is much greater than in our study (20).
In our study, bradycardia was more common in the dexmedetomidine group than in the fentanyl group. A severe bradycardia (less than 40 minutes) occurred in one patient of dexmedetomidine group that responded to atropine and intravenous fluid.
The frequency of cases with nausea and vomiting was 5 in the dexmedetomidine group and 2 in the fentanyl group. The lower rate of vomiting and nausea can be attributed to the lower dose of propofol in the dexmedetomidine group.
In the present study, oxygen saturation during colonoscopy was higher in the dexmedetomidine group than in the fentanyl group. The main reason was the lower consumption of propofol in group D than in group F.
The strength of the present study is its double-blind randomized clinical trial design. The most notable weakness of this study is that using dexmedetomidine requires more time than using fentanyl to have the most effect on pain killing, so it delayed the colonoscopy procedure. Another weakness of the present study is related to its small sample size.
5.1. Conclusions
The pain intensity during colonoscopy was lower in the dexmedetomidine group than in the fentanyl group. The rescue dose of Propofol was higher in the fentanyl group than in the dexmedetomidine group. The combination of dexmedetomidine and propofol provided a more appropriate analgesic result compared to fentanyl and propofol for colonoscopy.
Considering the risk of bradycardia and hypotension in our study and most other studies, we propose to examine the effect of dexmedetomidine and ketamine (to have a lower risk of bradycardia) in colonoscopy procedures.