1. Background
Caesarean section is a common method for pregnancy termination, the use of which is increasing annually. Some of the most important factors causing the increment include high maternal age, decrease in the number of deliveries, growing use of electronically monitored embryos, and possibility of elective cesarean section in breech deliveries. Today, spinal anesthesia has become one of the most common anesthetic procedures in cesarean section due to its many benefits such as early onset of breastfeeding in the operation room. Bupivacaine is now recognized as the most common drug in spinal anesthesia. Caesarean section requires high level of sensory block (T4) and this level of anesthesia requires a high dose of bupivacaine, which itself has side effects such as hypotension, nausea and vomiting, and prolonged recovery after surgery. Various studies have shown that simultaneous use of local anesthetic drugs and adjuvant drugs can enhance effectiveness and reduce the side effects of bupivacaine.
Dexmedetomidine, an alpha-2 adrenergic receptor agonist, is widely used to induce sedation in intensive care units (ICUs). This drug can simultaneously create sensory and motor block and is effective in improving visceral and cutaneous pain.
2. Objectives
Spinal anesthesia is today known as an elective method in abdominal and lower extremity surgeries due to its low cost, easy, fast, and effective operation, low complications, and most importantly, complete patient alertness during surgery (1). Studies in this area showed that with the improvement of community health, maternal mortality rates have decreased to half in the first half of the 20th century. However, with the growing prevalence of spinal anesthesia after the 1980s, mortality rate has significantly reduced and one of the most important reasons is improved safety and reduced complications following spinal anesthesia in cesarean section (2). However, spinal anesthesia is associated with complications such as short duration, hypotension, and bradycardia due to the blockage of the sympathetic system (3, 4).
Dexmedetomidine, an alpha-2 adrenergic receptor agonist, is widely used as a drug to induce sedation in ICUs. This drug was first used for intra-spinal cord injury in prostatectomy and can simultaneously create a sensory and motor block improving visceral and coetaneous pain. For this reason, it has been currently considered as one of the most effective drugs in spinal anesthesia (5-7).
Dexmedetomidine is more effective than clonidine in terms of analgesic effect, and it is associated with hemodynamic stability and better quality of anesthesia and analgesia during and after surgery with fewer side effects (7-9).
Regarding the combination of anesthetic drugs, one of the aspects that has been neglected is the study of the depth of sedation and analgesia during surgeries, especially cesarean sections where there are limits and considerations regarding inducing sedation and amnesia.
3. Methods
The study population consisted of women undergoing cesarean section in Shohada-e-Tajrish Hospital of Tehran, Iran, and their newborns. The subjects were selected randomly. The sample size was calculated based on the mean and CSI standard deviation of the mothers in both groups. The mean and standard deviation of neonatal Apgar score were not used because first of all there is no similar study measuring the Apgar score difference after intrathecal dexmedetomidine injection, and secondly, neonatal Apgar score is variable based on maternal conditions and is affected by various maternal factors. We used the previous study data to determine the sample size and we obtained the following numbers:
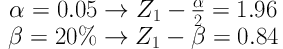
µ1 = CSI mean in dexmedmotidine group = 74,
µ2 = CSI mean in control group = 82,
S1= CSI standard deviation in dexmedmotidine group,
S2 = CSI standard deviation in control group,
Accordingly, the sample size was calculated at about 76 in each group:
This randomized double-blind study was performed after approving the title, registering the proposal, and obtaining a code of ethics from in Shohada-e-Tajrish Hospital in Tehran (code of ethics: IR.SBMU.FNM.REC.1396.2684).
Prior to the initiation of the study, a written informed consent was obtained from the patients who met the inclusion criteria.
The inclusion criteria consisted of maternal age between 20 and 25 years, no history of preeclampsia, no premature embryo, no twin gestations, no history of hypersensitivity or anaphylactic shock, and no spinal anesthesia contraindications.
The exclusion criteria comprised long duration of cesarean section leading to general anesthesia, massive hemorrhage, placenta accreta, increta and precreta during operation, or any other event that interrupts routine cesarean section and leads to non-routine interventions.
Patients were divided into two groups based on random number table. The case group received 10 mg of dexmedetomidine diluted in 4 cc of bupivacaine 0.5%, and the control group received 4 cc of bupivacaine 0.5% plus 1 cc of normal saline.
The volume of the anesthetic drug was reached to 3 ml by normal saline 0.9% in order to equalize the amounts of drugs used in the two groups. Each group received 3.5 cc of either BV or BVD solutions.
None of the patients received any premedication. Before starting anesthesia, IV line was accessed, and fluid therapy by normal saline 5 cc/kg was routinely performed. During the operation, standard fluid therapy was administered.
Monitoring during operation included the control of vital signs such as blood pressure, heart rate, and peripheral pulse oximetry to evaluate the hemodynamic status of the patients. Also, all the patients received 5 L of oxygen per minute by face mask during the operation.
3.1. Nerve Block
Spinal block was obtained in sitting position by a 25-gauge needle, and intrathecal injection was performed at a rate of 0.2 mL/s. After the procedure, the patients stayed in supine position. In cases that the sensory block did not reach to the sensory level of T6 after 20 seconds, spinal block was changed to general anesthesia.
3.2. Spinal Block Monitoring
The patients’ respiratory and hemodynamic parameters included mean blood pressure, heart rate per minute, respiratory rate per minute, and oxygen saturation before the block, immediately after the block, every two minutes during the operation till delivery, every 30 minutes till the end of the operation, and 180 minutes after the end of the operation. In cases where systolic blood pressure dropped to 20% of baseline and blood pressure or systolic blood pressure dropped to lower than 100 mmHg, sufficient fluid and incremental phenylephrine were injected. In cases where the heart rate of the patient reduced to below 55 or the patient had nausea and normal blood pressure, 0.5 mg atropine was injected.
In this study, we used the Ramsay Sedation Scale to assess the level of patients’ sedation during cesarean section. The amount of pain during operation and the need for postoperative analgesics were also measured by visual analogue scale (VAS). In cases where VAS was more than 4, diclofenac suppository was used to assuage pain. Also, in order to measure the depth of anesthesia, the BIS (as an electroencephalography-related monitoring) was used. Apgar score was also applied to evaluate the effect of bupivacaine and dexmedetomidine on neonates at birth and 5 minutes after birth by a pediatrician who was not involved in the study and was unaware of the group allocations.
4. Results
In this study, the data of 152 patients who were candidates for elective cesarean section under spinal anesthesia were evaluated in case (n = 76) and control (n = 76) groups. The demographic information of the two groups is presented in Table 1. There was no significant difference in demographic characteristics between the two groups.
| Variable | Control Group | Case Group | P Value |
|---|---|---|---|
| Age, y | 31.24 (6.90) | 30.51 (4.05) | 0.57 |
| Gestational age, week | 38.55 (0.77) | 38.38 (0.59) | 0.9 |
| ASA I/II | 63/13 | 66/10 | 0.497 |
| Duration of surgery, min | 42.89 (9.25) | 43.2 (8.6) | 0.71 |
a Values are expressed as mean (SD).
Hemodynamic status of the mothers during the operation (i.e., heart rate and blood pressure) was also measured (Tables 2 and 3).
| Mean Arterial Pressure, mmHg | Control Group | Case Group | P Value |
|---|---|---|---|
| 1 | 75.6 ± 6.5 | 75.2 ± 7.1 | 0.079 |
| 2 | 74.7 ± 7.1 | 74.1 ± 6.9 | 0.721 |
| 3 | 73.8 ± 6.8 | 74 ± 6.9 | 0.194 |
| 4 | 74.1 ± 4.1 | 74.8 ± 5.1 | 0.571 |
| 5 | 73.9 ± 7.8 | 73.7 ± 8.8 | 0.153 |
| Pulse Rate, Mean | Control Group | Case Group | P Value |
|---|---|---|---|
| 1 | 81.66 | 71.34 | 0.147 |
| 2 | 79.55 | 73.45 | 0.391 |
| 3 | 78.61 | 74.39 | 0.554 |
| 4 | 81.55 | 71.45 | 0.156 |
In this study, VAS criteria were measured in both groups after surgery and during recovery. Results indicated a significant difference in postoperative pain score between the two groups, showing that the control group experienced significantly less pain after the surgical procedure (P = 0.00; Table 4).
| Case Group | Control Group | P Value |
|---|---|---|
| 2.94 ± 1.19 | 1.00 ± 1.05 | 0.000 |
In addition, Apgar score was calculated to evaluate the health of newborns. There was no significant difference between the subject and control groups in 0, 5, and 10-minute Apgar scores (P = 0.033, P = 0.043, P = 1.00, respectively, Table 5).
| Apgar Score, Min | Control Group | Case Group | P Value |
|---|---|---|---|
| 0 | 8.15 ± 1.23 | 8.42 ± 1.79 | 0.295 |
| 5 | 9.89 ± 0.44 | 10 ± 0 | 0.043 |
| 10 | 10 | 10 | 1 |
We also calculated BIS every two minutes since birth and then every 15 minutes after birth until the end of the surgery. The results reflected a significant difference between the two groups in terms of BIS after 2 minutes of injection (Tables 6 and 7).
| Bispectral Index, Min | Control Group (n = 76), Mean | Case Group (n = 76), Mean | P Value |
|---|---|---|---|
| 2 | 93.76 ± 4.89 | 94.47 ± 2.19 | 0.250 |
| 4 | 88.98 ± 3.38 | 90.98 ± 4.36 | 0.002 |
| 6 | 83.52 ± 4.44 | 93.36 ± 3.15 | 0.00 |
| 8 | 81.56 ± 4.89 | 92.63 ± 3.56 | 0.00 |
| 10 | 81.92 ± 5.86 | 89.36 ± 2.85 | 0.04 |
| Bispectral Index, Min | Control Group (n = 76), Mean | Case Group (n = 76), Mean | P Value |
|---|---|---|---|
| 15 | 78.53 ± 2.00 | 94.79 ± 1.40 | 0.00 |
| 30 | 78.21 ± 2.14 | 94.05 ± 2.02 | 0.00 |
| 45 | 77.29 ± 8.56 | 94.42 ± 2.38 | 0.00 |
| 60 | 78.93 ± 3.82 | 94.53 ± 1.77 | 0.00 |
| 75 | 78.68 ± 3.01 | 93.42 ± 2.17 | 0.00 |
| Ramsay Scoremin, Min | Control Group, (n = 76), Mean | Case Group (n = 76), Mean | P Value |
|---|---|---|---|
| 15 | 2.08 ± 0.64 | 1.58 ± 0.49 | 0.00 |
| 30 | 2.21 ± 0.52 | 1.21 ± 0.41 | 0.00 |
| 45 | 2.08 ± 0.72 | 1.26 ± 0.44 | 0.00 |
| 60 | 2.21 ± 0.69 | 1.21 ± 0.41 | 0.00 |
| 75 | 2.25 ± 0.78 | 1.26 ± 0.443 | 0.00 |
Ramsay Sedation Scale has been used as a subjective numeric scale to evaluate mental state of patients. In this study, we found a significant difference between the groups, and the group in which intrathecal dexmedmotidine was used, ideal sedation was achieved (Table 8).
5. Discussion
Today, the use of α2-adrenergic receptor agonists is more widely accepted due to greater effects on analgesia during spinal anesthesia and hemodynamic complications. Dexmedetomidine, as an agonist drug of this family, is more effective on alpha 1 and 2 receptors than clonidine. Nowadays, this drug is known as one of the safest and most effective drugs due to its limited effects on the respiratory system and the level of patients’ consciousness during surgery (10, 11).
In the spinal cord, alpha 2c and alpha 2a adrenergic receptors directly reduce pronociceptive neurotransmitter release from primary neural terminals by hyperpolarization of spinal interneurons through dependent G protein potassium channels.
The primary outcome of this was the improvement of anesthesia indicator during and after surgery in the case group compared with the control group obtained following the addition of 7.5 μg of dexmedetomidine to 15 mg of bupivacaine hyperbaric in spinal anesthesia during cesarean section.
Different studies have shown that the use of dexmedetomidine can improve the quality of spinal anesthesia. Gupta et al. posited that the use of dexmedetomidine could prolong the duration of spinal anesthesia in abdominal and lower limb surgeries (12). Also, a similar result was obtained by Halder et al. in lower limb surgeries under spinal anesthesia (13).
Apgar is known as a health indicator. The type of selected anesthesia in cesarean section has always been influenced by the effects of the drug on neonatal Apgar scores at 0, 5, and 10 minutes after birth. The results indicated that co-administration of dexmedetomidine in the subject group had adverse effects on neonatal Apgar scores and there was no significant difference between the case and control groups at any of the measured minutes (14).
The effect of adding dexmedetomidine on spinal anesthesia during cesarean section and its ineffectiveness on neonatal Apgar scores had been confirmed in previous studies (15). In a study by Neuman et al. on patients who were candidates for cesarean section, it was shown that dexmedetomidine had no adverse effects on neonatal Apgar scores in these patients (16).
In another study performed by Palanisamy et al., the administration of dexmedetomidine, even intravenously, had no adverse effects on the maternal symptoms and Apgar scores in patients with spinal disorders (17). Also, Sun found that the effects of dexmedetomidine + bupivacaine were similar to those of bupivacaine + fentanyl, and there was no significant difference in Apgar score between the two groups (18).
Therefore, several studies have confirmed the use of dexmedetomidine in spinal anesthesia in cesarean section (19-21). Hypotension is known as a common complication of spinal block, which is usually controlled by intravenous fluids, phenylephrine, and ephedrine (22-27).
BIS is the first quantitative score measured by electroencephalography and is used clinically to evaluate depth of anesthesia. Different studies have been performed on the effect of simultaneous use of dexmedetomidine and bupivacaine on the duration of analgesia. In this study, there was a significant difference between the two groups in terms of BIS score. Likewise, Schneider et al. showed that BIS was in direct association with the type of drug used during anesthesia (28).
Chattopadhyay et al. proposed that dexmedetomidine could reduce BIS during abdominal surgeries, while there was a significant difference in recovery time between patients who received dexmedetomidine and the other patients (29). In another study undertaken by Morrison et al., the use of dexmedetomidine was associated with lower Ramsay scores (30). In spinal anesthesia, the effect of dexmedetomidine on Ramsay score has been investigated. NurKaya et al. proved that dexmedetomidine increases Ramsay score and the level of consciousness during surgery.
5.1. Conclusions
Our findings indicated that the simultaneous use of dexmedetomidine and bupivacaine in spinal anesthesia for cesarean section could have positive results such as lower postoperative pain, less need for sedative drugs, more suitable level of sedation during operation, and less negative effects on neonatal health as compared to bupivacaine alone.