1. Introduction
Churg-Strauss syndrome (CSS; or eosinophilic granulomatosis with polyangiitis) is a rare autoimmune disease. It is a systemic vasculitis associated with asthma and eosinophilia. Its incidence is estimated to be 2.5 cases per 100000 individuals, with a higher frequency among individuals with a history of asthma and is associated with blood and tissue eosinophilia (1). The lungs and skin are commonly affected yet it can affect other organs, including the heart, kidneys, nerves, and bowel. Some patients may develop severe or life-threatening complications, such as gastrointestinal involvement and heart disease, whereas others are only mildly affected with skin lesions and nasal polyps (2). The course of the illness ranges from subacute to fulminant, with most deaths due to complicating heart disease.
2. Case Presentation
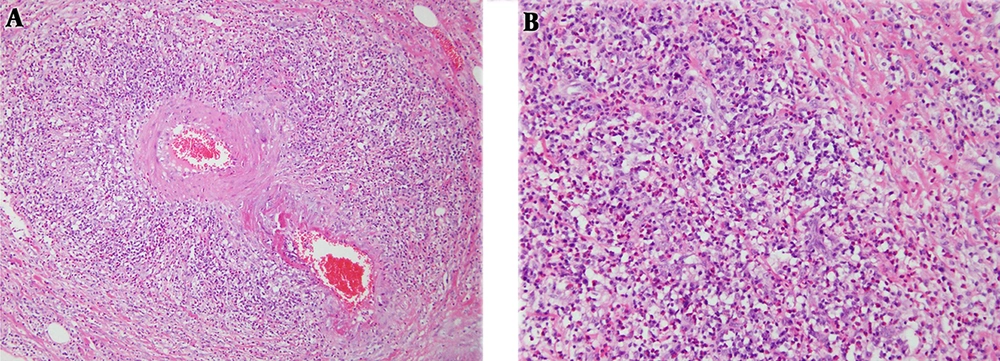
A 47-year-old man (height of 175.8 cm and weight of 74.8 kg) complained of a tingling sensation in his leg. He had a history of hypertension and asthma in the previous two years, and history of sinus surgery for sinusitis in the previous one year. Ten days before admission, the patient had a tingling sensation in his left leg, from the posterior thigh to the foot. His spinal radiography findings were unspecified. Therefore, the patient was suspected as having a disc herniation, and observation was decided while continuing the medical treatment. Four days before admission, the patient had a tingling sensation that progressed in both legs. The symptoms did not improve, and motor weakness progressed below the ankle. In the physical examination at admission, motor power was grade 5 in the hip flexor and knee extensor in both lower extremities. However, it was grade 0 in the ankle dorsiflexor, and first toe extensor and flexor, and grades 4 and 0 in the ankle plantar flexor on the right and left sides, respectively. Sensory loss was not found in the L1 - L4 dermatomes, yet was 50% on the right side and 0% on the left side for the L5 and S1 dermatomes. He also had a heating sensation in his left foot and no knee-jerk reaction. He showed a weight loss of 6 kg during the last month, and the signal intensity of the bone marrow on L-spine magnetic resonance imaging (MRI) was diffusively reduced to be less than or equal to the disc. These findings indicated a hematologic disease and the possibility of malignancy with little preserved fat marrow. Abdominal and pelvic computed tomography (CT) revealed diffuse wall thickening of the gallbladder with some irregularity. However, the bone marrow biopsy slides showed a generally hyper-cellular (50% to 60%) marrow for the patient’s age, with small hypo-cellular regions (0% to 20%). On aspirate smears, the eosinophil counts were markedly increased, with expanded eosinophil myelocytes and metamyelocytes. The results of the pulmonary function tests showed an obstructive pattern, such as a forced vital capacity of 54%, a forced expiratory volume in one second of 46%, and forced expiratory flow between 25% and 70% of 33% of the predicted values. The findings from a nerve conduction study suggested multiple mono-neuropathies. In addition, his laboratory findings showed peripheral eosinophilia and positivity for myeloperoxidase anti-neutrophil cytoplasmic autoantibody (MPO-ANCA; Table 1). Therefore, CSS was strongly suspected, and steroid and cyclophosphamide therapies were started after nerve biopsy under spinal anesthesia. At that time, the symptoms were progressing to the left wrist drop and both legs. However, nerve biopsy results were consistent with demyelinating peripheral neuropathy. The patient underwent cholecystectomy under general anesthesia to exclude gallbladder cancer, suspected on the basis of CT findings, and pathological confirmation was possible with a gallbladder specimen (Figure 1).
| Items | Test Results | Reference Range |
|---|---|---|
| WBC (eosinophil) | 21.6 (61.9) | 4.0 ~ 10.0 × 103/µL (0 ~ 5%) |
| Eosinophil count | 12330 | 50 ~ 500/µL |
| ESR | 45 | 0 ~ 9 mm/hr |
| IgM | 51 | 57 ~ 288 mg/dL |
| IgA | 227 | 84 ~ 438 mg/dL |
| IgG | 2207 | 680 ~ 1620 mg/dL |
| IgE (total) | 2500.0 | 0 ~ 100 IU/mL |
| HBsAg | Negative | Negative (< 1.0) S/CO |
| Anti-HCV | Negative | Negative (< 1.0) S/CO |
| ANA titeration | Negative | Negative (< 1:40) |
| ANCA (qualitative) | Negative | |
| ANCA (PR3 Ab) | Negative: 0.3 | |
| ANCA (MPO Ab) | 160.5 | |
| RF | Reactive | Nonreactive |
| Anti-CCP | 0.5 | < 5.0 U/mL: (-), ≥ 5.0 U/mL: (+) |
| RA (quantitative) | 27.0 | < 15.0 IU/mL |
Abbreviations: ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasm antibody; anti-CCP, citrulline antibody; anti-HCV, antibodies against hepatitis C virus; ESR, erythrocyte sedimentation rate; HBsAg, hepatitis B surface antigen; IgA, immunoglobulin A; IgE, immunoglobulin E; IgG, immunoglobulin G; IgM, immunoglobulin M; MPO, myeloperoxidase; RA: rheumatoid arthritis; RF, rheumatoid factor; WBC, white blood count.
3. Discussion
According to the American College of Rheumatology (ACR), the diagnostic criteria for CSS are as follows (3): (1) Asthma, (2) eosinophilia, (3) mono-neuropathy or polyneuropathy, (4) transient pulmonary infiltrates, (5) paranasal sinus abnormalities, and (6) histological evidence of extravascular eosinophils. Patients are diagnosed if at least four of the six ACR criteria for CSS are met. In this case, chest radiography revealed normal findings, and CSS was confirmed on the basis of five of six criteria that were met. The CSS usually manifests in three stages (4). The early stage is marked by airway inflammation. The formation of nasal polyps requires surgical removal, often more than once, and sinusitis may also be present. The second stage is characterized by inflammation of the gastrointestinal tract. The third phase is inflammation and damage of blood vessels. During this phase, patients with CSS may experience fever, weight loss, and lack of energy. Thus, the present case appeared to have been confirmed in the second to third phase.
Cardiac and neurological involvements are often observed; cardiac complications account for 50% of deaths (2). Early diagnosis and treatment will prevent organ damage and mortality. However, it is difficult to diagnose individual symptoms that occur separately. Although classified as vasculitis, ANCA positivity is observed in only 40% to 60% of patients. In addition, most patients will have elevated titers of perinuclear (p-) ANCA (5). Anti-myeloperoxidase activity (MPO-ANCA) level may also be elevated (6). Furthermore, ANCA, specifically MPO-ANCA, is a valuable adjunct in supporting the diagnosis of CSS yet is not useful for monitoring disease activity (7).
Most patients have a history of asthma, sinusitis, bronchitis, pneumonia, and hyper-allergic skin reactions (5). The most common neurological complications, which occur in approximately 20% of cases, are cranial nerve palsies, mono-neuropathy, including phrenic nerve, mono-neuropathy multiplex, or polyneuropathy (8). The course of the polyneuropathy may be acute and mimic Guillain-Barré syndrome (9). In this case, the patient initially complained of tingling sensation of unilateral leg, like disc herniation. In addition, symptoms progressed and a series of tests with mono-neuropathy multiplex showed suspicious malignancy. Therefore, it took a lot of time to perform the initial differential diagnosis and various evaluations were done.
The five-year survival rate for people with Churg-Strauss angiitis is estimated to be 66% to 100%. However, the morbidity is considerable when assessed by health-related quality-of-life measures. Treatment usually involves corticosteroids and cyclophosphamide. Effective treatment of CSS requires suppression of the immune system. The treatment duration is prolonged with induction of remission (steroids alone or in combination with cyclophosphamide), followed by maintenance treatment, sometimes lasting 12 to 18 months or longer (1). Fortunately, the patient began treatment before life-threatening organ invasion and responded well to medical therapy. Therefore, the medical team are currently observing him continuously as part of outpatient care.
In this case, the primary symptom was tingling sensation in the leg, like in disc herniation. The patient showed a rapid progression of symptoms with absence of initial muscle weakness. The five-year survival rate for people with CSS is estimated to be 66% to 100%. Therefore, early differential diagnosis and adequate treatment are important for patients with CSS.