1. Background
Post-operative pain management is of prime importance and can be performed through various methods (1). A wide range of medications including opioids, paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), gabapentin, pregabalin, tramadol, ketamine, and so forth, have been used alone or in combination to achieve this purpose (2-4). Beside systemic drugs, other anesthesia techniques including neuraxial techniques, transverse abdominis plane (TAP) block, wound injection, and intraperitoneal instillation have already been used for pain management after obstetric and gynecologic surgery (5-9). Paracetamol has limited effects to preclude and control post-caesarean pain (10). Ketorolac has been used to control post-operative pain, and its pharmacokinetics were reported safe for caesarean section (11, 12). Compared to opioids, non-narcotic analgesics such as paracetamol and ketorolac have less respiratory and awareness complications for newborn infants, but higher amounts of them are needed to produce adequate analgesia, if administered alone, which can cause adverse effects in the newborn. However, the use of multi-modal analgesia not only does give rise to sufficient pain relief after caesarean section, but also decreases the amount of the analgesic drug doses required for postoperative pain relief and their side effects (13). Dexmedetomidine is a selective central α2-adrenergic receptor agonist that has analgesic, sedative, and anxiolytic properties, and does not cause respiratory depression (14).
2. Objectives
This study aimed to evaluate the impact of adding dexmedetomidine to paracetamol and ketorolac in the i.v. PCA device on post-caesarean pain.
3. Methods
After receiving the institutional Ethics Committee’s approval (Ref: IR.IUMS.REC.1395.27278) and obtaining informed written consent, 60 parturient patients candidates for undergoing caesarean section in a university hospital were assigned in a randomized double-blind clinical trial. The sample size was estimated by:
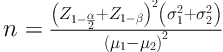
(d = µ1 - µ2 = 1.2), P = 90, α = 0.05
Parturients undergoing elective caesarean delivery under spinal anesthesia were recruited between January and June 2016 by double-blinded block randomization.
The study was registered in an international database (Ref: IRCT201601147984N24). The inclusion criteria comprised full-term pregnancy, the age of 18 - 38 years, ASA physical status I - II, elective caesarean delivery, primary or repeat caesarean section, Pfannenstiel incision, and being under spinal anesthesia. The exclusion criteria consisted of drug abuse, bleeding disorders, severe mental disorders, history of allergy to study drugs, gastrointestinal disease, obesity (BMI above 35), conversion to another method of anesthesia, pre-eclampsia and complications during the surgery.
Spinal anesthesia was established using hyperbaric bupivacaine (2.5 mL bupivacaine 0.5%, AstraZeneca, France). On arrival to the recovery room, parturients were randomly allocated to one of the two groups using a random number table. Participants and anesthesiologist performing pain assessments were blinded to group allocation. For post-operative pain management, an i.v. patient-controlled analgesia (PCA) device (Autofuser, ACE Medical Co., South Korea) was used for all patients in both groups. In the DP group, 3 µg kg-1 of dexmedetomidine (Precedex, Hospira Inc., USA) was added to 35 mg kg-1 of paracetamol (Apotel, Cobel Darou, Iran) up to 2 g, and in the DK group the same dose of dexmedetomidine was added to 1 mg kg-1 of ketorolac (Ketorolac, Exir, Iran). The PCA device was set to deliver a continuous infusion rate of 4 mL hr-1.
Patient assessment was done by a physician not aware of the PCA drugs at rest, 6th, 12th, and 24th hour after surgery. Pain score was obtained using a visual analog scale (VAS), (0 = no pain and 100 = worst pain imaginable). Ramsay sedation score (0- restless, 1- tranquil, 2- sleepy, 3- confused but responsive to verbal commands, 4- unresponsive to verbal commands, and 5- no response to painful stimuli), hemodynamic changes (blood pressure and heart rate), complications, patient’s characteristics, and satisfaction rate (exceeded expectation, matched expectation, and less expectation) were recorded.
When the pain score was greater than 30, meperidine (25 mg) was i.v. administered. Complications such as blood pressure and heart rate changes, nausea, vomiting, respiratory depression, bleeding, and dizziness were evaluated and treated, if identified.
The data collected were analyzed using the SPSS version 18 software. Employing the Kolmogorov-Smirnov test, the data were evaluated for normal distribution and accordingly, the Wilcoxon test was used for non-normal distribution data, t-test for normal distribution data, and the Fisher’s Exact test for variables with absolute values like the presence of special symptoms. Differences between the two groups were analyzed applying the Mann-Whitney test; the Wilcoxon test was used for intra-group analysis statistical comparisons with a Bonferroni correction. The Friedman test was employed to analyze the differences between pain assessment hours in the two groups. The qualitative data analysis was performed using the Chi-Square test and P values of < 0.05 were considered significant.
4. Results
Patient characteristic data, pain score, and analgesic consumption are given in Table 1. Pain scores at various times were meaningfully lower in the DK group than in the DP group. Although in the DP group, the rescue meperidine administration dose was higher during the first 24 hours, it did not lead to a significant difference between the two groups.
| Group DP | Group DK | P Value | |
|---|---|---|---|
| Age, y | 29.3 ± 5.1 | 30.8 ± 3.1 | 0.6 |
| Weight, kg | 77.04 ± 1.6 | 76.9 ± 11.9 | 0.4 |
| VAS | |||
| 0 h | 61.5± 12.5 | 59.4 ± 11.0 | 0.1 |
| 6 h | 50.7 ± 8.6 | 33.6 ± 8.9 | < 0.05 |
| 12 h | 35.7 ± 11.6 | 22.0 ± 7.6 | < 0.05 |
| 24 h | 24.0 ± 9.0 | 15.8 ± 6.6 | < 0.05 |
| Meperidine consumption, mg | 15.2 ± 0.5 | 12.5 ± 1.8 | 0.06 |
Patients Characteristics, Pain Score, and Meperidine Consumptiona
The Ramsay sedation score was 1 in both groups at 6, 12, and 24 hours during the post-operative period, with no difference between the two groups. The satisfaction rate was higher in the DK group than that in the DP group, which showed a statistically significant difference (Table 2).
With regard to complications, one from each group had dizziness and one patient in the DP group suffered nausea. Complications like vomiting, pruritus, sedation, hemodynamic changes, respiratory depression, bleeding, and gastrointestinal problems were not observed.
5. Discussion
In this study, the addition of dexmedetomidine to ketorolac provided a better analgesic effect than when it was added to paracetamol. A great number of studies have been carried out on post-caesarean pain management. In a study conducted by Parker and colleagues, the continuous intravenous infusion of opioid analgesics, compared to intermittent doses, gave rise to better analgesic effect within the first 24 hours after the caesarean section, and the satisfaction rate with pain control was higher (15). In this study, continuous infusion of non-narcotic analgesics had been used, rather than the intermittent bolus administration of analgesics, in caesarean section.
Paracetamol in combination with dexmedetomidine and an opioid has been used for creating analgesia. Paracetamol has analgesic and antipyretic effects, but it does not have the NSAIDs side effects such as peptic ulcer, platelet dysfunction, and cardiac and renal problems (16). The mechanism of action of paracetamol is to inhibit central cyclooxygenase-3, and thus reducing the production of prostaglandins in the central nervous system (17). It may also cause to moderate the serotoninergic inhibitory pathways and affect somewhat the opioid system and the N-acetyl-methyl-d-aspartate receptors (18).
The recommended dose of paracetamol in adults is 1 g intravenously maximally up to four times per day which, compared with opioids, is not likely to cause nausea, vomiting, and respiratory depression at such dosage. In addition, due to its different mechanism of action, compared to NSAIDs, it does not bring about platelet and renal dysfunction. Compared to NSAIDs and considering its fewer side effects, paracetamol is a more preferred choice for providing peri-operative analgesia (19). Swaika and co-workers proved in their study, compared to dexmedetomidine, paracetamol caused more analgesic effect in the laparoscopic cholecystectomy within the first 24 hours after the surgery (20).
In another study conducted by Liew and colleagues, following the single administration of the highest dose of paracetamol (4 g) to manage post-caesarean pain, approximately 5% of the medication was secreted into the breast milk. High-dose administration of paracetamol can be risky for both mother and newborn (21). In our study, the addition of dexmedetomidine to lower doses of paracetamol (2 g) not only did cause analgesia, but also reduced the incidence of complications.
Studies have demonstrated that the analgesic effect caused by ketorolac is similar to that of opioids, but it has fewer side effects and has also a ceiling analgesic effect, and often does not provide adequate analgesia when administered alone. In previous studies, by adding vitamin B complex, the effective dose of ketorolac was reduced by half to lower the chance of side effects (22). Gastrointestinal bleeding and acute renal failure are of the most prominent side effects of ketorolac. In the present study, adding dexmedetomidine to ketorolac not only reduced the dose of ketorolac and the associated complications, but also provided effective analgesia for pain management after caesarean section.
Ready and colleagues demonstrated that postoperative continuous infusion of ketorolac, compared to intermittent administration, decreases morphine consumption (23). Various studies indicate that the administration of ketorolac during lactation, compared to opioid, brings about no recognized side effect on the newborn owing to its inconsiderable amount of secretion in breastmilk (24). Moreover, the injection of ketorolac relieves post-caesarean pain and reduces opioid consumption (25).
Compared to clonidine, dexmedetomidine has a higher affinity to the α-2 adrenergic receptor, which makes it have more analgesic effects. It does not interact with the GABA system, it does not cause respiratory depression, and it produces cardioprotective effects by reducing central sympathetic tone.
In some studies, adding dexmedetomidine as an adjuvant to opioids or local anesthetics led to prolonged intraoperative analgesia, more postoperative pain relief, less nausea and vomiting, and fewer hemodynamic changes (26-28). In a study of pain management after caesarean section, adding dexmedetomidine to sufentanil decreased opioid consumption and increased patient satisfaction (29). In the present study, dexmedetomidine was added to non-opioid analgesics (paracetamol and ketorolac) to avoid opioid infusion and the side effects thereof on both mother and newborn. In patients who are resistant to the effects of opioids, the addition of dexmedetomidine can be useful in controlling the pain (30). Bradycardia and hypotension may occur with dexmedetomidine, thus much care should be taken in setting its infusion dose rate to prevent cardiovascular complications. Sedative and analgesic effects of dexmedetomidine are due to the stimulation of α2-adrenergic receptors in the core part of the locus coeruleus (31). In this study, no cardiovascular complication requiring treatment was observed with the administered dose of dexmedetomidine.
To recapitulate, the results of this study indicate that for post-caesarean pain management, adding dexmedetomidine to lower than usual doses of non-opioid analgesics (paracetamol and ketorolac) can cause appropriate analgesia without producing any considerable complications. In addition, although there was no significant difference, meperidine consumption was slightly higher in the paracetamol group, and in contrast, the satisfaction rate was significantly higher in the ketorolac group than in the paracetamol group. Therefore, it is recommended to undertake further studies with other dosages of these analgesics to achieve more effective drug combinations.


