1. Background
Obstruction of the airway by a large glottic mass imposes a great challenge to most anesthesiologists during intubation, especially when tracheostomy is not available. Therefore, awake intubation using fiber-optic bronchoscope is the safest method to obtain a secured airway (1).
Anesthetic management of awake fiber-optic intubation (AFOI) should consider comfort of patients and intubation circumstances (2). The optimal technique for AFOI should make the patient cooperative and comfortable, and also provide blunted airway reflexes, especially if there is difficult laryngeal anatomy and/or pathology (3).
It may be difficult to choose appropriate sedative drugs due to their depressant effects on respiration (4). Sedatives such as benzodiazepines, sevoflurane, remifentanil, ketamine, propofol, and dexmedetomidine are frequently utilized throughout AFOI (3).
Propofol is a strong sedative hypnotic drug characterized by rapid onset and recovery due to its lipid solubility with amnestic and anti-emetic properties. On the other hand, it has a respiratory depressant and dose-dependent hypotensive effect, which limits its employment (5).
Ketamine has many advantages as it possesses amnestic and analgesic properties, preserves muscle tone and airway reflexes, in addition to maintaining spontaneous breathing. However, some anesthesiologists prefer not to employ it for sedation due to its sympathomimetic effects, excessive salivation, and vomiting (6).
Ketamine and propofol combination (ketofol) has many benefits since ketamine reduces consumption of propofol and provides hemodynamic stability, while propofol relieves hallucinations associated with ketamine (7). Therefore, the employment of this combination has many advantages such as preserving the airway patency, keeping the patient spontaneously breathing along with stabilizing hemodynamic profile and quick recovery (8).
Dexmedetomidine is a highly selective α2-adrenergic agonist, which reduces endogenous norepinephrine release in the brain and spinal cord (9). It is employed as a sedative in many procedures (e g., cataract surgery (10)) without inducing respiratory depression. In addition, it is effective and safe when used in awake intubation with flexible fiber-optic bronchoscope (11).
2. Objectives
The current prospective, randomized study aimed at comparing the effects of administering either dexmedetomidine-propofol or ketofol for AFOI in terms of intubation conditions, hemodynamic stability, and patients and anesthesiologist satisfaction.
3. Methods
The current prospective, randomized, double-blind study was conducted from June 2016 to March 2018. The study protocol was approved by the Ethics Committee of Tanta University hospitals, Tanta, Egypt (30663/12/15) and registered at the Pan African Clinical Trials Registry (PACTR201701001959580). After obtaining written informed consent, 80 patients of either gender, aged 18 - 60 years, ASA I-III, and difficult airway intubation due to laryngeal mass who were candidates for laryngeal mass biopsy under general anesthesia were enrolled in the study. Patients with bleeding disorders, nasal mass, allergy to any of the drugs under study, uncontrolled systemic diseases with excessive use of analgesics or long term sedative medication and the ones that were uncooperative or refused to give informed consent were excluded from the study.
Patients were randomly assigned 1:1 to administer one of the two different sedative infusions for AFOI, dexmedetomidine-propofol (group D; n = 40) or ketofol (group K; n = 40). The randomization was done using a computer-generated random numbers concealed in sealed envelopes showing the group assignment. A nurse, who was blind to the study and data gathering, made group assignments by reading the number in the envelope. A senior anesthesiologist, who was blind to the group identities, performed AFOI in all subjects. An anesthesiologist, who was blind to the randomization, prepared the drugs. Also, the observer was entirely blind to the groups or medication administered to the patients.
A preoperative visit one day prior surgery was performed. History taking, systemic examination (extensive airway examination and the difficulty of intubation assessed both by the clinical examination and indirect laryngoscope), and preoperative investigations were performed. All patients were ordered to fast for at least six hours before the procedure. Patients were informed about the technique of AFOI with sedation, as well as their required collaboration.
Patients received standardized premedication in the form of diazepam tablet 10 mg and ranitidine tablet 150 mg given with sips of water two hours before surgery and intramuscular atropine
0.01 mg/kg was administered 30 minutes before application of topical anesthesia.
Once in the operating theatre, standard monitoring was performed with the aid of pulse oximetry, electrocardiography, and noninvasive arterial blood pressure, and 100% oxygen (2 L/minute) was delivered via nasal cannula.
Preparation of the nasal mucosa was achieved by instillation of 0.1% xylometazoline hydrochloride nasal drops and nasal packing was performed using cotton-tipped swabs soaked in 2% lidocaine and epinephrine (1:200000) solution. The more patent nostril during nasal packing was selected for nasal intubation. A 10% lidocaine was sprayed onto the oral cavity to decrease the gag reflex.
The medications were infused via 50-mL syringe pumps (Injectomat Agilia IS, Fresenius, 018090/22716658Brezins, France) labeled as I, II, III, and IV. Syringe I contained dexmedetomidine (2 mL DEX (200 μg) added to 48 mL of 0.9% saline solution; concentration of 4 μg/mL) to be infused at a rate of 1 μg/kg for the first 10 minutes as intravenous bolus dose followed by infusion of 0.5 μg/kg/hour; syringe II contained ketofol in a ratio of 2:1 ( 20 mL of 1% propofol added to 2 mL ketamine (50 mg/mL) plus 28 mL of 0.9% saline; concentration of 4 mg/mL propofol and 2 mg/mL ketamine) and an initial loading dose of 0.125 mL/kg over 10 minutes was given followed by the infusion of 0.125 mL/kg/hour; syringe III contained 0.9% normal saline, while syringe IV contained propofol (20 mL of 1% propofol added to 30 ml of 0.9% saline; concentration of 4 mg/mL) to be infused at the same rate of dexmedetomidine and ketofol, respectively. Patients in the D group received I and IV infusions, while patients in the K group received II and III infusions.
Patient’s sedation level was evaluated using Ramsay sedation scale (RSS) throughout the whole procedure (1: anxious, agitated or restless; 2: cooperative, oriented, and tranquil; 3: sedated, but responds to command; 4: asleep with brisk response to stimulus; 5: asleep with sluggish response to stimulus; and 6: asleep with no response). If RSS was < 3 at any time during the procedure and if the patient or the anesthetist were uncomfortable, rescue propofol doses (20 mg increments) were given.
3.1. Fiberoptic Intubation Technique
When sufficient level of sedation (RSS ≥ 3) was obtained, tracheal intubation was conducted using fiber-optic endoscope (Karl Storz, 1130 1BN1, Germany). Epidural catheter inserted in the suction channel of fiber-optic endoscope was used to produce modified topical anesthesia of the airway as described by Liu et al. (3), by the spray-as-you-go technique onto the glottis and below vocal cords using 2% lidocaine. Once entering the trachea, the endotracheal tube was slided on the bronchoscope, and intubation was confirmed using capnography. The endotracheal tube was fixed, infusion of the study drugs was discontinued and general anesthesia was induced to all groups using propofol 1 mg/kg, fentanyl 1 µg/kg, and atracurium 0.5 mg/kg. The maintenance of anesthesia was performed using a balanced anesthetic technique of isoflurane in oxygen, and incremental doses of atracurium (0.1 mg/kg). At the end of the operation, extubation was performed after neuromuscular block reversing with neostigmine (0.05 mg/kg) and atropine (0.01 mg/kg). The patients were then transferred to recovery room and closely monitored for 24 hours.
3.2. Outcome Variables
The primary outcome variable was time to reach sufficient sedation level defined as the interval between the drug infusions and RSS ≥ 3. Secondary outcomes were as follows:
-Intubation time (time from insertion of the fiberoptic bronchoscope throughout the nose till confirmation of intubation with capnography) and number of intubation trials
-Number of patients in need of rescue propofol in each group
-Patient’s discomfort score (0: no discomfort; 1: probable mild discomfort, no patient resistance; 2: restless patient, minimal patient resistance; 3: restless patient, severe patient resistance)
-Patient’s tolerance to endoscopy and intubation on a five-point scale (1: no reaction, 2: slight grimacing, 3: heavy grimacing, 4: verbal objection, and 5: defensive movement of head and hands)
-Cough score (1: no cough, 2: slight cough (no more than two coughs per sequence), 3: moderate cough (3 - 5 coughs per sequence), 4: severe cough ( > 5 coughs per sequence)
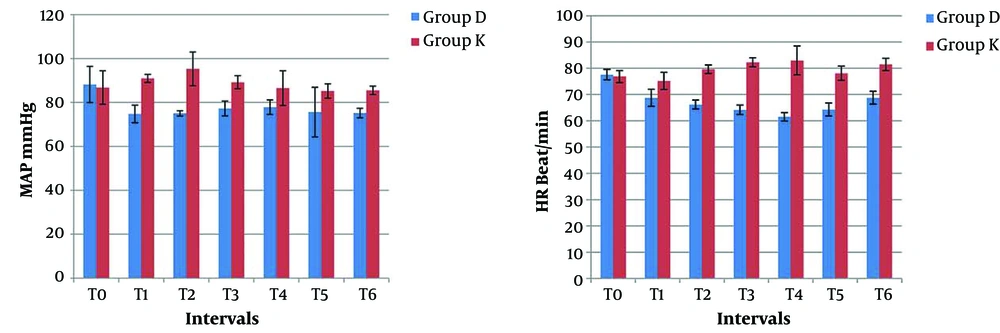
-Heart rate (HR) and mean arterial blood pressure (MAP) were recorded at the following points: on arrival to the operating room as a baseline (T0), after bolus sedation immediately prior to fiberoptic intubation (T1), at the passage of the fiber-optic endoscope through vocal cords (T2), at the time of intubation (T3), then at 1, 3, and 5 minutes after intubation (T4, T5, T6, respectively). Hypotension, defined as reduction in MAP < 20% of baseline measurement, was managed by intravenous fluid and bolus dose of 5 mg ephedrine, repeated if necessary. Bradycardia, defined as HR < 60 beat/minute, was treated with 0.01 mg/kg atropine.
-Airway obstruction was evaluated by airway obstruction score (1: patent airway; 2: airway obstruction relieved by neck extension; 3: airway obstruction requiring jaw thrust)
-Hypoxic episodes (SpO2 < 92%) or apnea (cessation of spontaneous breathing for more than 20 seconds) were recorded and managed by airway support and assisted ventilation using bag and mask technique.
-Patient’s satisfaction 24 hours post-operation (very satisfied, satisfied, dissatisfied, very dissatisfied)
-Anesthesiologist’s satisfaction score (1: excellent, 2: good, 3: fair, 4: poor) was also recorded.
Failure of AFOI, due to developing severe resistance during the procedure, was considered as the study failure, and induction was performed with standard doses of propofol, fentanyl, and rocuronium; then, intubation was performed with the fiberoptic bronchoscope and the availability of a cricothyrotomy set as well as the ear, nose, and throat team ready to perform emergency tracheostomy if needed.
3.3. Statistical Analysis
Sample size calculation suggested a minimum of 37 patients in each group based on the results of a previous study (12) to detect a significant reduction in time to reach RSS ≥ 3 of at least 2.2 minutes at α error of 0.05, standard deviation of 2.6, and the study power of 95%. Therefore, 40 cases were enrolled in each group to overcome possible dropouts. SPSS version 16 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analysis. Normality of data was assessed using the Shapiro-Wilk test. Numerical variables were compared between the two groups utilizing the Student independent t test for data with normal distribution or by the Mann -Whitney U test, if otherwise. Categorical variables were presented as patients’ number and percentage and analyzed by the Chi-square or the Fisher exact test where appropriate. P value < 0.05 was considered significant.
4. Results
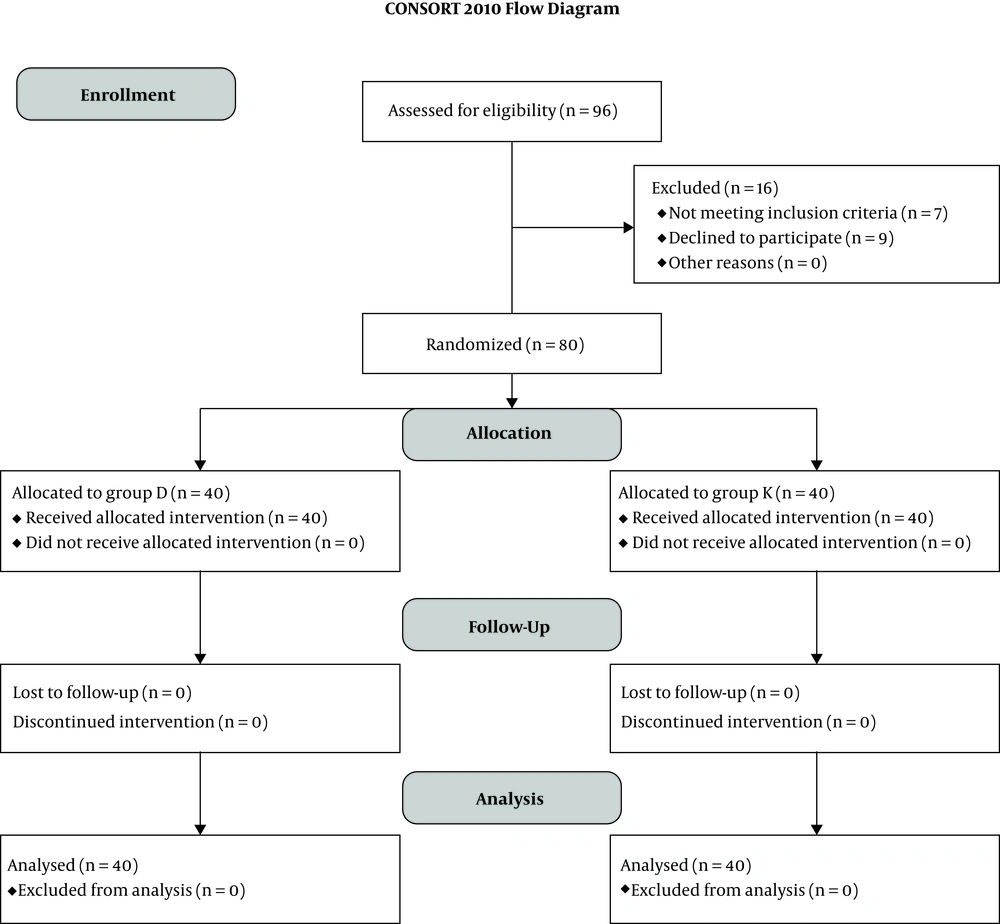
Out of 96 patients evaluated for eligibility, seven patients met the exclusion criteria (four patients had uncontrolled systemic disease, one patient had bleeding disorder, and two patients had excessive analgesic usage), nine patients refused to participate in the study, and the remaining 80 patients were randomly allocated into one of the two groups (n = 40) (Figure 1).
There were no significant differences between the groups in terms of demographic variables (age, gender, body weight, and ASA (the American Society of Anesthesiologists) physical status) (Table 1).
| Variable | Group D | Group K | P Value |
|---|---|---|---|
| Age, y | 0.185 | ||
| Mean ± SD | 54.52 ± 9.66 | 57.38 ± 9.41 | |
| Range | 36 - 73 | 35 - 81 | |
| Gender | 0.807 | ||
| Male | 29 (72.5) | 27 (67.5) | |
| Female | 11 (27.5) | 13 (32.5) | |
| Weight, kg | 0.191 | ||
| Mean ± SD | 76.83 ± 9.29 | 74.22 ± 8.31 | |
| Range | 61 - 96 | 54 - 95 | |
| ASA physical status | 0.944 | ||
| I | 17 | 19 | |
| II | 18 | 14 | |
| III | 5 | 7 |
a Values are expressed as mean ± SD or No. (%).
All patients in the current study reached the targeted sedation level (RSS ≥ 3) and had successful AFOI. However, time to reach RSS ≥ 3 and intubation time were significantly shorter (P = 0.000*) with fewer number of intubation attempts in the K group compared to those of the D group. Moreover, the number of patients that needed rescue doses of propofol was also significantly less in group K (P = 0.035*). The patients’ discomfort score and their tolerance to endoscopy and intubation were comparable between the two groups, while better scores were observed in the group K, but with insignificant difference (P-values = 0.132, 0.137, and 0.211, respectively). No statistically significant difference in cough scores were observed between the two groups (P = 0.611). Cough developed in nine and 12 patients in groups D and K, respectively (Table 2).
| Variable | Group D | Group K | P Value |
|---|---|---|---|
| Time RSS ≥ 3 min | 12.575 ± 0.675 | 6.825 ± 0.781 | 0.000b |
| Intubation time, min | 24.63 ± 1.17 | 16.48 ± 1.43 | 0.000b |
| Number of intubation trials [median (IQR)] | [2 (1 - 2)] | [1 (1 - 1)] | 0.003b |
| Number of patients needing rescue propofol | 19 (47.5) | 9 (22.5) | 0.035b |
| Patient’s discomfort score | 0.132b | ||
| 0 = No discomfort | 8 (20) | 15 (37.5) | |
| 1 = Probable mild discomfort, no patient resistance | 11 (27.5) | 14 (35) | |
| 2 = Restless patient, minimal patient resistance, | 19 (47.5) | 10 (25) | |
| 3 = Restless patient, severe patient resistance | 2 (5) | 1 (2.5) | |
| Patient’s tolerance to endoscopy | 0.137 | ||
| 1 = No reaction | 9 (22.5) | 17 (42.5) | |
| 2 = Slight grimacing | 13 (32.5) | 14 (35) | |
| 3 = Heavy grimacing | 12 (30) | 6 (15) | |
| 4 = Verbal objection | 5 (12.5) | 3 (7.5) | |
| 5 = Defensive movement of head and hands | 2 (5) | 0 (0) | |
| Patient’s tolerance to intubation | 0.211 | ||
| 1 = No reaction | 5 (12.5) | 10 (25) | |
| 2 = Slight grimacing | 11 (27.5) | 15 (37.5) | |
| 3 = Heavy grimacing | 19 (47.5) | 14 (35) | |
| 4 = Verbal objection | 1 (2.5) | 0 (0) | |
| 5 = Defensive movement of head and hands | 4 (10) | 1 (2.5) | |
| Cough score | 0.611 | ||
| 1 = No cough | 31 (77.5) | 28 (70) | |
| 2 = Slight cough | 9 (22.5) | 12 (30) | |
| 3 = Moderate cough | 0 (0) | 0 (0) | |
| 4 = Severe cough | 0 (0) | 0 (0) |
Abbreviation: IQR, interquartile range.
a Values are expressed as mean ± SD, median (IQR), or No. (%).
b Statistically significant difference.
At baseline measurement (T0), MAP and HR changes in the two groups were comparable (P-values = 0.433 and 0.136, respectively). There was a statistically significant difference between the study groups regarding the changes in hemodynamic parameters at various points of measurements after infusion of the study medications. Patients in group D had statistically significant lower MAP and HR after the loading dose till five minutes after intubation (from T1 to T6) (P = 0.000*). Furthermore, a statistically significant decrease was observed between baseline values and subsequent measurements of MAP and HR in group D (P = 0.000*). Since the findings were significant, no interventions were required (Figure 2).
Adverse respiratory events were infrequent in the study, only four patients in group D and eight patients in group K had airway obstruction grade II, relieved by neck extension and this had no statistically significant value (P = 0.348). In addition, no hypoxic episodes (SpO2 < 92%) or apneic attacks were noted. Patients’ satisfaction levels were similar in the two groups (P = 0.687). However, the anesthesiologist’s satisfaction scores in the group K were higher than those of the group D (P = 0.013*) (Table 3).
| Group D | Group K | P Value | |
|---|---|---|---|
| Patient’s satisfaction | 0.687 | ||
| Very satisfied | 18 | 21 | |
| Satisfied | 12 | 12 | |
| Dissatisfied | 9 | 7 | |
| Very dissatisfied | 1 | 0 | |
| Anesthesiologist’s satisfaction | 0.013b | ||
| 1. Excellent | 10 | 23 | |
| 2. Good | 20 | 11 | |
| 3. Fair | 7 | 6 | |
| 4. Poor | 3 | 0 |
a Values are expressed as No. (%).
b Statistically significant difference.
5. Discussion
The current study results showed that ketofol and dexmedetomidine-propofol combination were suitable and satisfactory for AFOI. However, ketofol provided more satisfactory conditions for AFOI than dexmedetomidine; demonstrated by less time to reach the targeted sedation level (RSS ≥ 3), shorter intubation time with fewer numbers of intubation trials, and less need of rescue dose of propofol in the ketofol group as compared to those of the dexmedetomidine group. Moreover, patients receiving ketofol had low discomfort score with better tolerance to endoscopy and intubation as well as better hemodynamic profile than the ones receiving dexmedetomidine. Although, patients’ satisfaction was similar in the two groups, anesthesiologists’ satisfaction was higher in the ketofol group.
The current study results supported the findings of a study by Sruthi et al. (13), comparing ketofol with dexmedetomidine sedation for outpatient transesophageal echocardiography. They reported that the time to achieve RSS ≥ 3 was significantly shorter in the ketofol group than the dexmedetomidine group. Both agents had a stable respiratory profile with no rescue sedation required in the two groups. Their results showed that the patient satisfaction score in the groups was comparable, while the ketofol group showed higher scores compared to the dexmedetomidine group.
Yagan et al. (12), in their study on sedation in cataract surgery found that ketofol, compared with dexmedetomidine, had a faster onset of sedation. They found similar results for patients and surgeons satisfaction scores. However, they demonstrated a significant decrease in MAP after drug administration in the two groups compared with baseline values; while for HR, the decrease was observed only in the dexmedetomidine group, although the difference between the groups was insignificant. Furthermore, Hassan (14) showed lower MAP when dexmedetomidine was compared to ketofol in endoscopic retrograde cholangiopancreatography and suggested that combining ketamine with propofol caused better hemodynamic stability and decreased the incidence of hypotension.
Dexmedetomidine causes hypotension by central sympatholytic action via α2B receptors. Other involved mechanisms were: blocking norepinephrine release from presynaptic sites by inhibiting neuronal discharge from the locus ceruleus in the brainstem. In the heart, bradycardia via a vagomimetic effect may happen with sympatholytic effect by inhibition of tachycardia (cardioaccelerator nerve inhibition) (15).
In the current study, the intubation time was significantly shorter in the ketofol group compared with that of the dexmedetomidine-propofol group. This might be attributed to the more sustained and deeper sedation provided by ketofol. Hence, patients in the ketofol group had lower discomfort scores as well as better tolerance to endoscopy and intubation. Although there was no statistical significance when the two groups were compared in terms of these scores, it was practically interpreted by the fact that ketofol provided more comfortable and favorable conditions for AFOI, compared with dexmedetomidine-propofol, leading to less number of intubation attempts with significantly shorter intubation time in the ketofol group than the dexmedetomidine-propofol group. No studies compared the two medications during AFOI; however, a study by Liu et al. (3), comparing dexmedetomidine and fentanyl, as sedatives during AFOI, demonstrated a significantly shorter intubation time in the fentanyl group compared with the dexmedetomidine group; they suggested that this is due to less patient restrain in the fentanyl group.
There were infrequent adverse respiratory events in the two groups. Fewer patients with grade 2 respiratory obstruction were detected in the dexmedetomidine group with no statistically or clinically significant differences and no other adverse respiratory effects in the two groups.
A different study by Daabiss et al. (16), compared different concentrations of ketofol for short procedural sedation; they observed a mild increase in EtCO2 (mild respiratory depression) in all groups. Canpolat et al. (17), added ketamine to either propofol or dexmedetomidine, and found that both combinations were effective in relieving severe anxiety, when used for deep sedation of uncooperative children during tooth extraction. Nevertheless, ketofol ensured higher surgeon satisfaction with less nausea and vomiting. They concluded that ketofol in children patients may be a better drug for tooth extraction.
Furthermore, Willman and Andolfatto (18), found that in emergency department, it was easy to use mixture of ketamine and propofol in one syringe for sedation and analgesia effectively, and it was accompanied by high degree of satisfaction among patients and physicians.
The current study had some limitations. First, the patient population was small, further studies with large population are needed. Second, there was no control group to detect differences between ketofol and other sedative agents. Further studies should compare different concentrations of ketofol to find the safest and lowest effective dose of ketofol for AFOI.
Finally, the current study concluded that the administration of either dexmedetomidine- propofol or ketamine-propofol combinations for sedation during AFOI was associated with adequate intubation conditions with ketofol producing faster onset of sedation, shorter intubation time, stable hemodynamic profile as well as less propofol requirement, higher anesthesiologist’s satisfaction without increased side effects when compared with dexmedetomidine-propofol.