1. Background
Propofol-related infusion syndrome (PRIS) has recently been recognized as a critical condition (1-3). Many case reports and studies have been reported in the fields of pediatric anesthesia, postoperative sedation, and the ICU. Furthermore, two case reports (4, 5) and a review article (6) included not only infants and children (4) but also adults (5, 6). This highlights the importance of paying attention to the development of this complication with an extended duration of total intravenous anesthesia (TIVA) as well.
Regarding the pathophysiological mechanism of this condition, it is believed to be as a result of injury to the mitochondrial electron transport chain and the resultant metabolic disorders that are caused by both propofol agents and the lipid solvent (7-14). However, opinions are not consistent regarding the effects on liver cells (15-18). Two studies (15, 16) showed that propofol did not damage liver cells, while two other pieces of research (17, 18) reported that liver cells were damaged by the propofol reagent. Accordingly, the mechanisms and causative factors of PRIS have not been fully elucidated.
Hence, we designed this study to investigate PRIS and its prevention. PRIS induces serious pathophysiological changes and symptoms such as acute refractory bradycardia leading to asystole, metabolic acidosis, rhabdomyolysis, hyperlipidemia, and enlarged or fatty liver. However, so far, no established treatment has been provided. Since it is a relatively rare condition, thus establishing a simple research model using cultured cells might be very helpful for further investigations into this condition. Therefore, we performed experiments to determine whether the use of the C2C12 cell culture model is feasible for further investigation of the mechanisms of PRIS.
2. Objectives
To evaluate the possibility of a simple research model using cultured cells for fundamental research of PRIS, we collected basic data as a starting point for elucidation of the mechanism of PRIS. The aim of this study was to examine and evaluate the cell impairment effects of propofol reagents, which consisted of propofol, as the active ingredient along with a lipid solvent, using murine muscle cells that were differentiated from myoblast cells (C2C12 cells).
3. Methods
3.1. Cell Culture and Preparation of the Reagents
Murine C2C12 myoblast cells were cultured with 10 mL Dulbecco modified Eagle medium (DMEM) with Phenol red, 10% Fetal Bovine Serum (FBS) and 1% Penicillin and Streptomycin (PS) (all these chemicals are manufactured by FUJIFILM Wako Pure Chemical Corporation®, Osaka, Japan) in 10-cm cell culture dishes (Costar® Corning™, ME, USA). After confirmation of over 80% cell confluence on the culture dishes, differentiation of the cells was induced by the addition of DMEM with 2% Horse Serum (HS) and 1% PS every 2 days (19). Hence, cell culture plates with six wells (Costar® Corning™, ME, USA) were used as culture dishes. Each plate was filled with 3 mL DMEM with 2% Horse Serum (HS) and 1% PS. After 10 to 21 days from the beginning of differentiation, formation of muscle cells was determined by microscopic observation (TMS® Microscope, Nikon, Tokyo, Japan) of features such as fusion and enlargement of these cells.
Next, we performed two separate experiments to study the cytotoxic effects of propofol and its lipid reagent on the differentiated myoblast cell line. In the first experiment, the cultured muscle cells were separately exposed to the chemical reagent 2,6-diisopropylphenol (2,6 DIP) and the solvent, which was dimethyl sulfoxide (DMSO) [liposoluble organic compound (solvent), Sigma-Aldrich®: product number D2650], to individually assess the cytotoxic effects of the active reagent and the solvent. In the second experiment, the cultured muscle cells were separately exposed to commercially available 1% propofol and 10% soybean, at several concentrations as the test chemicals in the first experiment, to assess the effects of the propofol preparation that was used clinically.
In the experiment 1-a, culture mediums of differentiated C2C12 cells were changed and exposed to the cultured mediums (DMEM with 2% HS and 1% PS), which contained chemical reagents of 2,6 DIP (at concentrations of 0 (as the control), 0.1, 0.3, 1, 3 and 10 µg/mL), calculated by molecular weight (MW178.27), and stirred gently by slowly turning the culture dishes. In experiment 1-b, the cultured differentiated cells were exposed to culture medium containing DMSO at the same volume as in the corresponding 2,6-diisopropylphenol subgroups.
In the experiment 2-a, the culture mediums were changed and exposed to the cultured mediums (DMEM with 2% HS and 1% PS), which contained a commercially available 1% propofol (at concentrations of 0 (as the control), 10, 30, 100, 300 and 1000 µg/mL). In experiment 2-b, the differentiated muscle cells were exposed to a culture medium (DMEM with 2% HS and 1% PS) containing lipid reagents (10% Soybean) at the same volume as in the corresponding 1% propofol subgroups in the experiment 2-a. Eight cell culture dishes were used for each concentration of each reagent tested in the experiments 1 and 2, and 5 culture dishes were used for the control and propofol 100 µg/mL groups in the experiment 3.
3.2. Experiment 1-a
Propofol component, as the chemical reagent 2,6 DIP (Sigma-Aldrich®: product number D126608), was mixed with the cell culture medium at six concentrations: 0 (control), 0.1, 0.3, 1, 3, and 10 µg/mL. After 60 hours incubation with 2,6 DIP, the whole cells in the dish were collected using 0.3 mL of 0.25% trypsin (FUJIFILM Wako Pure Chemical Corporation®, Osaka, Japan), which, the live cells, dead adherent cells, and dead floating cells were peeled from the dishes with gentle pipetting. The rate of cell death was investigated by trypan blue staining (0.4% Trypan blue solution: FUJIFILM Wako Pure Chemical Corporation®, Osaka, Japan), since dead cells absorb trypan blue into their cytoplasm while living cells do not. Trypan blue staining was performed by the usual method as the following. Suspended cells were examined following gentle mixing in a 1:1 ratio with trypan blue solution. Then the number of dead cells and living cells were counted using a manually operated counter (TYPE-TM® TOHO™, Tokyo, Japan) and a Burker-Turk hemocytometer (Sunlead Glass™, Koshigaya, Saitama, Japan). After counting, the cell death rate was calculated by dividing the number of dead cells by the total number of cells.
3.3. Experiment 1-b
Dimethyl sulfoxide (DMSO) [liposoluble organic compound (solvent)] was used instead of the 2,6 DIP reagent at the same concentrations and solvent volumes as to the propofol reagent in the experiment 1-a.
After incubation of 60 hours, the rate of cell death was investigated by trypan blue staining like the experiment 1-a.
3.4. Experiment 2-a
Commercially available 1% propofol solution for animal experiments (Mylan Inc.®, Osaka, Japan) was used for this experiment. The solution was diluted with the same culture medium as was used for differentiation of the C2C12 cell culture (i.e. DMEM with 2%HS and 1%PS,), to obtain the following six concentrations of propofol: 0 µg/mL (the control), 10, 30, 100, 300, and 1000 μg/mL. The commercially available propofol was incubated with the medium for 48 hours at 37ºC under exposure to 5% CO2 in the incubator. The incubation time was shortened from 60 hours in the experiment 1-a and 1-b to 48 hours in the experiment 2-a and 2-b for approaching the shorter time because continuous propofol infusion over 48 hours should be avoided for the prevention of PRIS. After 48 hours, the supernatant fluid of the cell culture medium was extracted and the number of floating cells was measured using a Coulter Counter (Z1 Coulter® Particle Counter, Beckman® Company, Atlanta, GA, USA) to estimate the cell death.
3.5. Experiment 2-b
A lipid reagent (10% Soybean, Wako®: Product no. 190-03776) was added to the culture medium (2% HS and 1% PS in DMEM) and diluted with the same volume as in the corresponding 1% propofol diluted solutions in the experiment 2-a and incubated for 48 hours at 37ºC under 5% CO2 in an incubator. Then the supernatant fluid in the cell culture medium was gathered and the number of floating cells, as a reflection of cell death, were measured using the same Coulter Counter as in the experiment 2-a.
3.6. Experiment 3
JC-1, a test using fluorescent reagent, was performed to investigate the propofol-induced mitochondrial disorder. After the incubation of the differentiated myoblasts with 100 μg/mL propofol, 2% HS, and 1% PS in DMEM for 48 hours, the cells were treated as the propofol group. On the other hand, after the incubation of the differentiated myoblasts without propofol, with 2% HS, and 1% PS in DMEM for 48 hours, the cells were treated as the control group. The mitochondria of the propofol and control groups were stained with the fluorescent dye JC-1 (Sigma-Aldrich™, St. Louis, MO, USA). The procedure was as follows: after discarding the cell culture medium with or without propofol 100 µg/mL, each well was washed twice with Krebs-Ringer solution (300 µL/well). Then JC-1 solution (15 µM) was added to each well and incubated for 10 minutes. Thereafter, 2 mL of DMEM with L-glutamine and HEPES without phenol red (FUJIFILM Wako Pure Chemical Corporation®, Osaka, Japan) was added to the wells and mitochondrial staining by JC-1 was observed using an inverted fluorescence microscope (Ti-E® Nikon™, Tokyo, Japan).
The images obtained by the microscope were analyzed by ImageJ software (NIH: National Institutes of Health, USA) for the assessment of red and green fluorescence. The JC-1 dye exhibits mitochondrial membrane voltage-dependent accumulation, which is indicated by a fluorescence wavelength shift from green (about 529 nm) to red (about 590 nm). Consequently, the decreased mitochondrial membrane potential is expressed as a reduction in the ratio of red to green (20).
Data are expressed as mean ± standard error of the mean (SEM) (Figures 1 and 2) or standard deviation (SD) (Figure 3) and were analyzed with two-way repeated measures ANOVA, post hoc Tukey’s multiple comparison test, Pearson correlation coefficient, and Student t-test (GraphPad Prism®, SPSS®) for cell death rate, number of floating cells, and comparison of fluorescence between red and green in the JC-1 experiment. P < 0.05 was considered statistically significant.
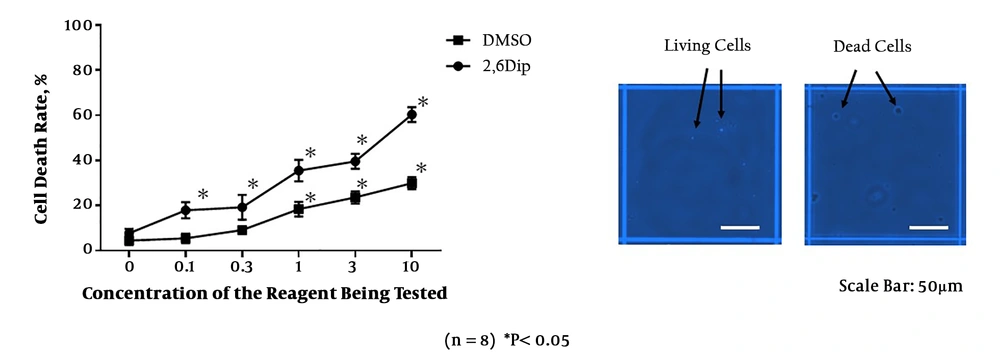
Cell death rate (%) assessed by trypan blue staining, following the incubation with different concentrations of 2,6-diisopropylphenol (2,6 DIP: chemical propofol reagent) or DMSO. The right panel shows the dead cells that absorb trypan blue into the cytoplasm and living cells whose cytoplasm was not stained.
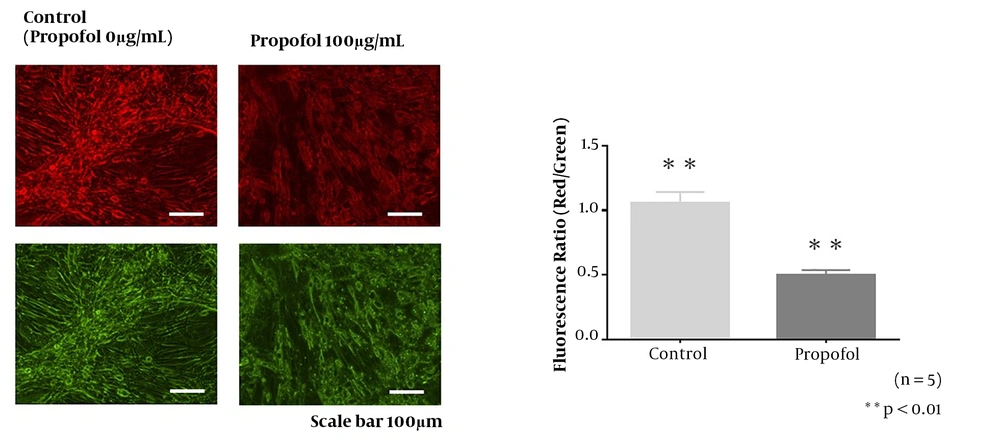
The JC-1 staining of mitochondria in the control group (0 µg/mL propofol) and 100 µg/mL propofol group. In the cells incubated with 100 µg/mL propofol for 48 hours, cell shrinkage, atrophy, and mitochondrial deformation were recorded. The decreased red/green fluorescence ratio in the propofol group suggests low mitochondrial membrane potential.
4. Results
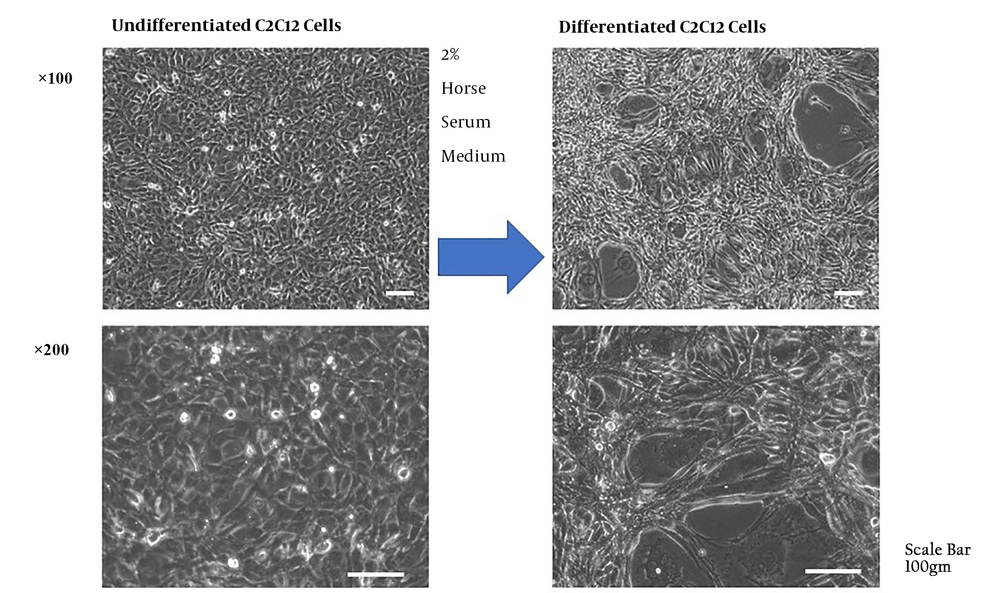
C2C12 cells (Murine myoblast cells) were cultured and differentiated into skeletal muscle cells by the addition of DMEM with 2% HS and 1% PS every 2 days for over 10 days (Figure 4).
Differentiation of C2C12 cells was induced by the addition of DMEM with 2% horse serum and 1% penicillin and streptomycin every 2 days for over 10 days. The differentiation was observed as the fusion of the myoblasts and formation of multinucleated myotube cells. The cells gradually transformed into thicker and longer cells.
4.1. Experiments 1-a and 1-b
Trypan blue staining showed an increase in cell death rate with increasing concentrations of 2,6 DIP (Figure 1). As in the experiment 1-a, the rate of cell death was also increased with elevating concentrations of the organic compound of DMSO (Figure 1).
The values of r2 (r Squared) in the Pearson correlation coefficient analyses were 0.816 in the 2,6 DIP group and 0.730 in the DMSO group. Significant differences were observed between 2,6 DIP and DMSO groups, except for the control concentration (Figure 1). The rates of cell death were significantly higher in the 2,6 DIP group compared with DMSO group. These findings suggested the possibility of cell toxicity with both 2,6 DIP and DMSO. Inter-concentration comparisons in the 2,6 DIP and DMSO groups showed that cell death rates in the 0.1, 0.3, 1, 3, and 10 μg/mL of 2,6 DIP subgroups were significantly higher than the control 2,6 DIP subgroup. In addition, cell death rates in the DMSO subgroups that corresponded to the 1, 3, and 10 μg/mL of 2,6 DIP subgroups were significantly higher than those in the control DMSO subgroup.
4.2. Experiments 2-a and 2-b
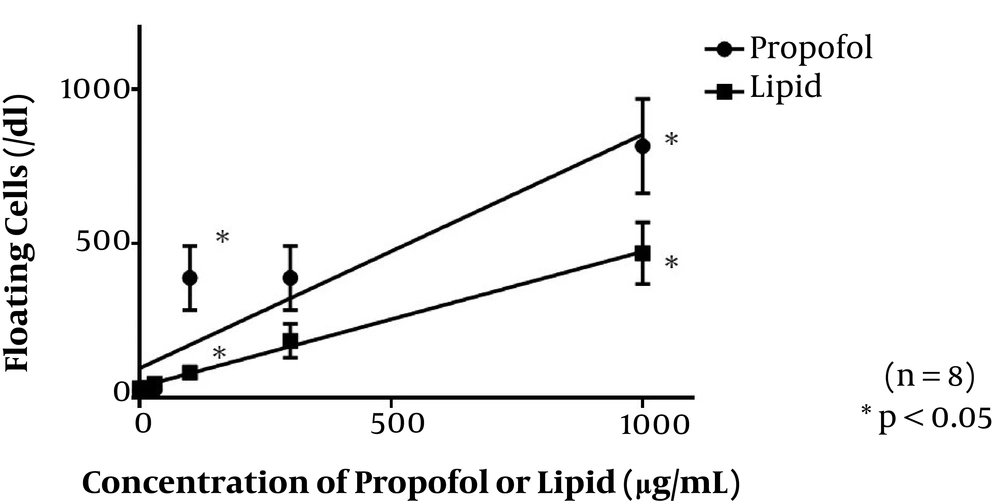
Addition of propofol solution to the cell culture led to an increase in the rate of floating dead cells with increasing concentrations of commercially available propofol (Figure 2). This indicated the cellular toxicity of prolonged exposure of muscle cells to propofol. By the addition of lipid emulsion solution alone to the cell culture as well, the rate of floating dead cells was increased with elevating the lipid concentration (Figure 2).
The values of r2 in the Pearson correlation coefficient were 0.855 in the propofol group and 0.997 in the lipid group. Significant differences were recognized between the propofol 100 and 1000 μg/mL subgroups and the corresponding lipid subgroups. In inter-concentration comparisons of the propofol and lipid groups, cell death rates in the 100, 300, and 1000 μg/mL propofol subgroups were significantly higher than those in the control propofol subgroup. In addition, the value in the highest lipid concentration subgroup, corresponding to 1000 μg/mL propofol, was significantly higher than in the control lipid subgroup. These findings suggested that the lipid emulsion itself, especially in high doses, was also cytotoxic to murine muscle cells.
4.3. Experiment 3
In the morphological observation of mitochondria by JC-1, cell shrinkage, atrophy, and deformation of mitochondria were presented in Figure 3, indicating the mitochondrial toxicity of the propofol reagent to muscle cells derived from the murine myoblast C2C12 cell line. The decreased red/green fluorescence ratio in the propofol group suggests low mitochondrial membrane potential (20). This result suggests that the effect of propofol-induced cellular and mitochondrial dysfunction is related to the deterioration of mitochondrial membrane potential.
5. Discussion
Propofol is a commonly used anesthetic for the induction of general anesthesia, maintenance of TIVA, and various examinations and treatment under sedation (21, 22). This reagent has been studied extensively and can be used in many different situations, including in patients with a family history of malignant hyperthermia (23). In recent years, PRIS is recognized as a critical condition that can cause fatal rhabdomyolysis (1-3). In fact, the use of propofol for prolonged postoperative sedation in pediatric patients has been warned against by the US Food and Drug Administration (FDA) (24). Moreover, adult cases of PRIS have also been reported (5, 6). Hence, investigation of detailed mechanisms and development of effective treatment strategies sound worthwhile (4-6).
As mentioned earlier, PRIS causes myocyte injury and rhabdomyolysis via dysfunction of muscle cell mitochondrial electron transport. However, the trigger for its onset and its genetic predisposition are still unknown (7-14). Since the occurrence of PRIS is considerably rare and investigations using human and animal models are difficult. Thus a basic and simple experimental model is needed to investigate the detailed mechanism of its occurrence and possible treatments. Hence, experiments using cultured cells have been reported in recent years (25, 26).
The current study demonstrated the cytotoxicity of propofol and lipid reagents toward muscle cells using a basic, relatively simple experiment, involving murine cell lines. Our results are in accordance with previous studies related to PRIS due to propofol-induced muscle cell injury (7, 25-27). Furthermore, our experiments used differentiated muscle cells derived from murine myoblast cells, which are similarly susceptible to cell damage as pediatric tissues. Therefore, this experimental model was supposed to be useful for further investigation to verify some treatments related to protecting mitochondria function.
In the current study, incubation with the animal use propofol reagent in experiment 2-a, which has the same composition as the human reagent, led to definite cellular injury following incubation at a concentration of 100 μg/mL (approximately 30 times the concentration used clinically in humans) for 48 hours. In the incubation experiment with the lipid reagent alone, which is used as a solvent in the propofol reagent, the same dose-dependent cell toxicity was observed. The staining experiment with JC-1 suggested mitochondrial pathological degeneration with prolonged exposure to propofol, as evidenced by the decreased mitochondrial membrane potential in the propofol 100 μg/mL group.
Our findings might offer a beacon of hope for the prevention and treatment of PRIS in the future. For example, this model might be useful for evaluation of the treatment of PRIS with mitochondrial coenzyme supplementation and the activating reagent of autophagy and mitophagy (mitochondrial autophagy), which support the cell’s self-cleaning system.
Nevertheless, our experimental model has some limitations. Since our experimental model involved only murine muscle cell cultures instead of human tissues and only basic research techniques, the results cannot be directly generalized to human tissues. Furthermore, the influence of genetics, blood flow, and the intracellular and extracellular fluids was not evaluated. Although the results of the chemical reagent in the experiments 1-a and 1-b are noteworthy so that this model might be applicable for mechanism clarification of PRIS, further studies are necessary for the future. Even though our results corroborated previous knowledge about PRIS such as the dose dependency of the toxic effects of propofol and the cell toxicity of the lipid reagent, the possible utility of our model, as an experimental model of PRIS, is suggested.
5.1. Conclusions
We investigated the mechanism of propofol-induced cell injury using a murine skeletal muscle cell model. The dose-dependent cell damage induced by the propofol reagent and a lipid solvent suggests the potential of this model as a basic experimental model for further elucidation of PRIS.