1. Background
In the preoperative period, children frequently endure significant stress due to the high psychological and physical demands of medical procedures. Their reactions to the preoperative period can be traumatic and often involve anger, fear, depression, and anxiety (1). To determine anxiety in children, Kain et al. developed the Yale preoperative anxiety scale modified (m-YPAS) (2). Another tool is the pediatric anesthesia emergence delirium (PAED) scale, created to identify delirium episodes in the postoperative period (3). These episodes are most frequent at first 30 minutes for distressed children on recovery.
The benefits of gabapentinoids (gabapentin and pregabalin) are well established in adults (4); however, only is gabapentin available for children of all ages (5). Gabapentin is a good candidate as it has shown to reduce anxiety in children with neurological deficits and gastrointestinal stress (6). It may cause some side effects, e.g. dizziness, diarrhea, and ataxia; however, cardiovascular and hematopoietic systems usually remain undisturbed (7, 8). In pediatrics, gabapentin induces fewer side effects and drug interactions than carbamazepine and phenytoin for refractory epilepsy (9). Furthermore, gabapentin has positive results for post-amputation neuropathic (10) and oncologic pain treatment in children (11). There are few studies in pediatrics investigating the use of gabapentin in the perioperative period, while no study was found on oncologic contexts for analgesia and sedation.
2. Objectives
We aimed to investigate the use of gabapentin in procedural sedation and analgesia as adjuvant therapy on oncologic pediatric patients.
3. Methods
A prospective, randomized, and double-blinded trial was conducted at Albert Sabin Infant’s Hospital from August 2017 to June 2018 in Fortaleza, Brazil. The study was approved by the local research ethics committee and registered by code NCT03681574. Written informed consent was obtained from all legal guardians before study enrollment. Children aged 1 - 6 years from both genders were submitted to lumbar puncture and/ or myelogram. All patients in this study either were diagnosed with cancer or were under investigation. The exclusion criteria were the presence of renal, pulmonary, heart or hepatic insufficiency, neurological disease, chronic use of anticonvulsants, or known allergy to the protocol medicine.
The oncologic procedures (lumbar puncture and myelogram) were indicated to diagnose and treat cancer in those children, to drain cerebrospinal fluid (diagnosis of cancer invasion in the central nervous system), or to administrate intrathecal chemotherapy (methotrexate protocol). The most frequent diagnostic cancer in our institution was acute lymphoblastic leukemia, as in other institutes.
The patients were sorted into three groups including the placebo group, 15 mg/kg gabapentin group (15 mg/kg GABA), and 30 mg/kg gabapentin group (30 mg/kg GABA). We used gabapentin salt (Sigma-Aldrich, Brazil Ltda®) to make gabapentin syrup. One to two hours before the procedure, each patient received orally strawberry flavored syrups labeled from 01 to 03 with compositions known only for pharmacists. The syrups were unmasked after statistical analysis by pharmacists. The dosage used was 0.3 mL/kg at a maximum of 12 mL. All patients received anesthetic local cream to reduce surgical puncture pain and they were accompanied by their parents in a child-adapted waiting room to reduce distress.
Before the procedure, the anesthetist prepared all patients following a pure inhalation anesthesia protocol of sevoflurane at 8% (induction) and 4% (maintenance) plus 50% N2O and FiO2. The anesthetic induction time was assessed in seconds through recording of the patient loss of consciousness and of corneal reflex; at this time, we evaluated the ended-expired sevoflurane. Intraoperatively, all patients received antiemetics (ondansetron intravenous 0.2 mg/kg every four hours). After the procedure, the patients were kept in observation in the recovery room. The researcher applied the CHIPPS pain scale and, if it scored more than 4 points, 20 mg/kg dipyrone up to 6/6 hours was given.
All patients were observed for changes in the m-YPAS at baseline (before drug administration), one hour after syrup administration, at separation from parents, and at anesthetic induction time (2). After the procedure, all patients were observed for changes in the PAED scale (this scale is the sum of 5 categories that which one could be 0 to 4; these categories are “eye contact”, “actions are purposeful”, “aware of surroundings”, “restless” and “inconsolable” and in the children and infants postoperative pain scale (CHIPPS-this scale is a sum of 5 categories, which categorie coulb be 0 to 2, as crying, facial expression, leg position, trunk position, restlessness, total of 0 to 10 points, ≥ 4 points indicated analgesic need). Scores were assessed at baseline (right after the procedure) and 30 minutes after the procedure finished in both scales (3, 12). The m-YPAS, PAED, and CHIPPS total scores of above 30, 10, and 4 points, respectively, indicated worse patient outcomes. Also, all patients were evaluated for the occurrence of vomits over eight hours after the procedure.
3.1. Statistical Analysis
The sample size was determined as 45 randomized patients. To do descriptive analysis, we calculated the average, standard deviation, and median for parametric data and interquartile range and range of values for nonparametric data. Students t-test, Mann-Whitney test, and chi-square test were applied for univariate analysis. For inter-group comparisons, we applied unpaired t test (parametric data) and Kruskal-Wallis test (nonparametric data). Post hoc Dunn test was used for non-Gaussian distributions. We considered an alpha of 0.05 as the level of significance. Analysis and graphic presentations were made by SPSS 22.0 software (SPSS Inc., Chicago, IL, USA).
4. Results
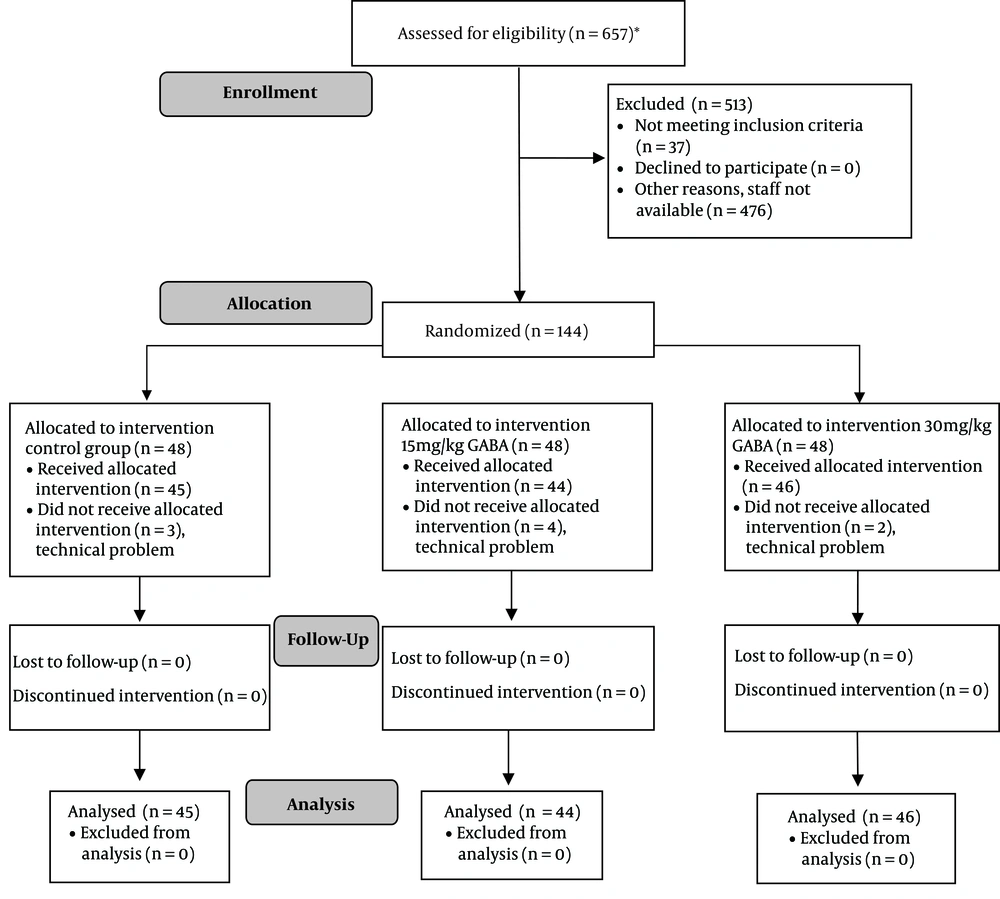
A total of 135 patients were analyzed, as shown in the CONSORT flow diagram (Figure 1).
4.1. General Characteristics and Patient Distribution
A total of 135 patients were enrolled in this study. Intrathecal methotrexate chemotherapy was administered to 82 patients within the procedure, from whom 103 were evaluated for vomiting. Demographic and clinical data on the day of the experiment did not show any distributive discrepancy between the placebo, 15 mg/kg GABA, and 30 mg/kg GABA groups (Table 1).
| Variables | Trial Groups | P Value | ||
|---|---|---|---|---|
| Placebo | 15 mg/kg GABA | 30 mg/kg GABA | ||
| GenderA | 0.772 | |||
| Male | 29 (64.4) | 29 (65.9) | 27 (58.7) | |
| Female | 16 (35.6) | 15 (34.1) | 19 (41.3) | |
| AgeB, y | 3.15 ± 1.47 (1 - 5.9) | 3.29 ± 1.42 (1-6) | 3.42 ± 1.39 (1 - 6) | 0.647 |
| WeightB, kg | 16.26 ± 5.43 (3.5 - 32.1) | 16.47 ± 4.36 (9 - 27) | 16.67 ± 4.12 (10 - 32.1) | 0.848 |
| Number of previous procedures | 0.082 | |||
| 0 - 3 | 30 (66.7) | 23 (53.5) | 20 (43.5) | |
| ≥ 4 | 15 (33.3) | 20 (46.5) | 26 (56.5) | |
| Type of procedureA | 0.935 | |||
| Myelogram | 15 (33.3) | 12 (27.3) | 16 (34.8) | |
| Lumbar puncture | 26 (57.8) | 28 (63.6) | 25 (54.3) | |
| Myelogram + lumbar puncture | 4 (8.9) | 4 (9.1) | 5 (10.9) | |
| ChemotherapyA | 0.991 | |||
| Yes | 27 (60.0) | 27 (61.4) | 28 (60.9) | |
| No | 18 (40.0) | 17 (38.6) | 18 (39.1) | |
aValues are expressed as mean ± SD or No. (%).
bThe general characteristics of patients (gender, age, weight), type of procedure, frequency of procedure, and the use of chemotherapy on the day of experiment did not show any distributive discrepancy between the placebo, 15 mg/kg GABA, and 30 mg/kg GABA groups (P > 0.05 based on chi-square test (χ2) and Kruskal-Wallis test).
4.2. Gabapentin Groups Presented m-YPAS Scores Lower Than the Placebo Group Only at Separation and Anesthetic Induction Time Points
The m-YPAS was used at baseline, one hour after syrup administration, separation of the patient from the guardian, and anesthetic induction time points. The m-YPAS scores of above 30, which indicated a pathological level of agitation, were observed in the mean scores of all groups. Although 15 mg/kg GABA (mean score of 60.30 ± 29.22) and 30 mg/kg GABA (mean score of 58.54 ± 32.44) groups had lower scores of m-YPAS than the placebo group (mean score of 80.96 ± 26.58) at separation (Table 2), they were calmer at the moments of higher stress levels once they were under the gabapentin effect. The same results were observed at induction when comparing placebo (mean score of 84.37 ± 20.00) and gabapentin groups (15 mg/kg GABA mean score of 65.36 ± 26.28 and 30 mg/kg GABA mean score of 60.86 ± 26.45). We found no difference between 15-mg/kg and 30-mg/kg GABA groups at separation and induction times, only at baseline, which would not be clinically relevant (supplementary material).
| Groups | m-YPAS | P Value* | |||
|---|---|---|---|---|---|
| Baseline | After One Hour | At Separation | At Induction | ||
| Placebo | 32.12 ± 20.04 | 32.23 ± 20.56 | 80.96 ± 26.58 A,B | 84.37 ± 20.00 C, D | < 0.001c |
| 15 mg/kg GABA | 35.34 ± 19.27 | 32.70 ± 20.51 | 60.30 ± 29.22 A | 65.36 ± 26.28 C | < 0.001c |
| 30 mg/kg GABA | 31.21 ± 19.48 | 30.71 ± 21.12 | 58.54 ± 32.44 B | 60.86 ± 26.45 D | < 0.001c |
| P value** | 0.094 | 0.205 | < 0.001cA, B | < 0.001cC, D | |
aValues are expressed as mean ± SD.
bWhen compared to placebo, patients who received gabapentin before the procedure had lower m-YPAS mean scores at separation from parents and induction of anesthesia using sevoflurane (P A, B, C, D < 0.001). There were no differences between placebo and gabapentin groups at baseline and after one hour of syrup administration. Statistical tests included * Friedman test, ** Statistical tests included Kruskal-Wallis test followed by post hoc Dunn’s test. A Placebo versus GABA 15 mg/kg at separation time, B Placebo versus GABA 30 mg/kg at separation time, C Placebo versus GABA 15 mg/kg at induction time, D Placebo versus GABA 30 mg/kg at induction time.
cSignificant P values < 0.05.
4.3. The Time of Induction and Percentage of Ended-Expired Sevoflurane Used Were Lower in the Gabapentin Groups
The time of anesthetic induction in the surgical room was lower in patients who received gabapentin; 15 mg/kg GABA group had a mean time of 55 seconds (± 28.0) and 30 mg/kg GABA group had a mean time of 49 seconds (± 22.0) at one to two hours before the procedure when compared to the placebo group that had a mean time of 88 seconds (± 30.0) (supplementary material). This result was also observed in the percentage of ended-expired sevoflurane needed to induce anesthesia; the placebo group had a mean value of 5.59% (± 0.43), the 15 mg/kg GABA group had a mean value of 5.07% (± 0.47), and the 30 mg/kg GABA group had a mean value of 4.97% (± 0.43). Gabapentin variation in doses (15 versus 30 mg/kg) had no significant difference in the time of induction or the amount of ended-expired sevoflurane inhaled.
4.4. Gabapentin Groups Had Lower PAED and CHIPP Scores Than the Placebo Group
Postoperatively, patients were assessed right after the procedure (time 0) and 30 minutes after the procedure finished (time 30) for delirium occurrence (PAED scale) and pain (CHIPPS scale). Patients who received gabapentin syrup (15 or 30 mg/kg) one to two hours before the procedure presented lower PAED and CHIPP scores than the placebo group at time 30 (Table 3) (P < 0.001). The placebo group had a mean score of above 10 (mean: 10.33 ± 6.11) on the PAED scale and this was particularly important for delirium occurrence, thus indicating the presence of delirium in need of medical intervention. As for the patients who received gabapentin syrup, none presented postoperative delirium (PAED score < 10) (15 mg/kg GABA mean: 2.6 ± 4.94 versus 30 mg/kg GABA mean: 2.63 ± 5.06). Even though there were significant differences in CHIPP scores between the placebo and gabapentin groups, the placebo CHIPP scores were lower than 6, indicating the absence of pain in need of pharmacologic intervention (placebo mean: 2.42 ± 2.45 versus 15 mg/kg GABA mean: 0.63 ± 1.43, and 30 mg/kg GABA mean: 0.65 ± 1.85). We found no significant differences between the GABA 15 mg/kg and GABA 30 mg/kg groups (supplementary material).
a15 and 30 mg/kg gabapentin groups had lower PAED and CHIPP mean scores than the placebo group at time 30 (p a,b < 0.001). PAED scores of above 10 were presented in the placebo group. All gabapentin patients had PAED scores of below 10. PAED and CHIPP mean scores at time 0 were not shown because patients were always sedated at this point.
bA, C Placebo versus GABA 15 mg/kg in A PAED and C CHIPP scores. B, D Placebo versus GABA 30 mg/kg in B PAED and D CHIPP scores.
cStatistical tests used were Kruskal-Wallis test followed by post hoc Dunn’s test.
dSignificant P values < 0.05.
4.5. Children with Less Than Three Prior Procedures Were More Likely to Benefit From Gabapentin
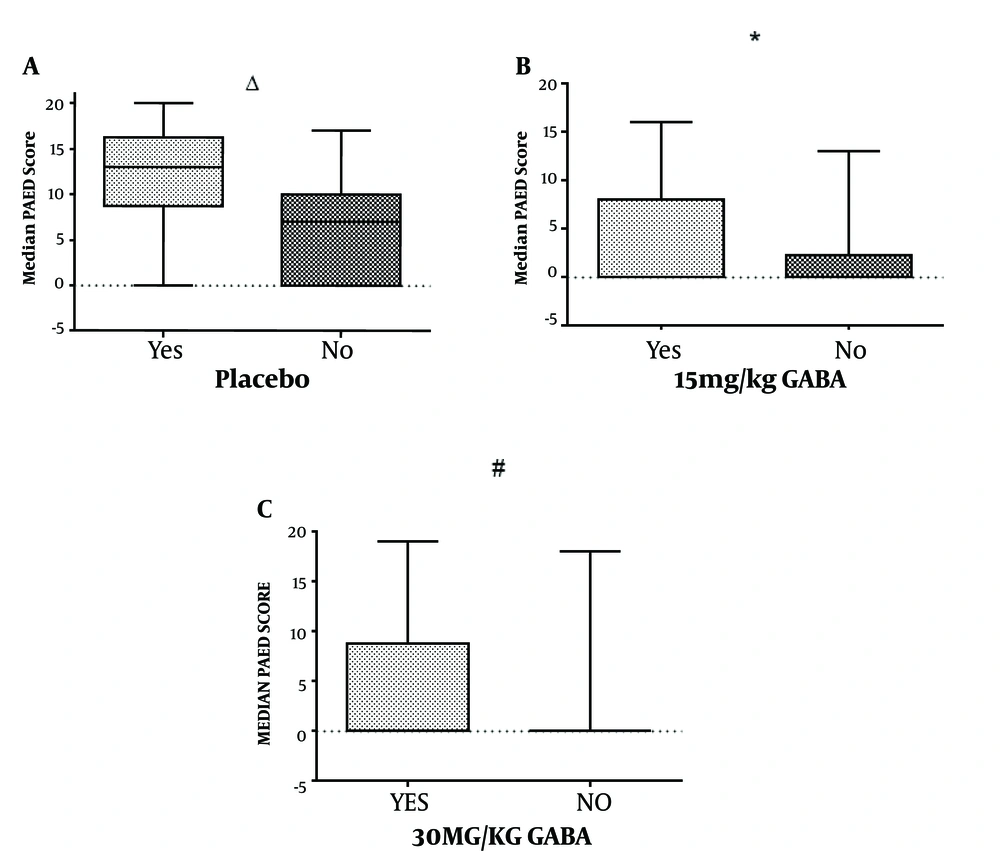
We compared median PAED scores in the placebo, 15 mg/kg GABA, and 30 mg/kg GABA groups according to the number of similar prior medical procedures, since its frequency could affect the sensitivity of children to the lumbar puncture or myelogram. Overall, children who had more than three medical procedures had higher median PAED scores than those who did not (Figure 2). However, when comparing only within the 15 mg/kg GABA group, there was no significant difference concerning similar prior procedures (P < 0.05). Besides, all gabapentin subgroups had median PAED scores of lower than 10. This could indicate the benefit of gabapentin as an adjuvant anesthetic, especially in children with less than three prior medical procedures.
PAED median scores. Placebo group versus Gabapentin groups in children who had more than three prior similar medical procedures. Patients who received 15 mg/kg gabapentin syrup (15 mg/kg GABA) and underwent less than three prior medical procedures had no significant difference in median PAED scores from those with more than three prior medical procedures of the same group (P = 0.488). We found higher PAED median scores in subgroups that had more than three prior medical procedures within the placebo and 30 mg/kg GABA groups. Δ > Three prior medical procedures versus < three prior medical procedures in the placebo group (P value = 0.006). * > Three prior medical procedures versus < three prior medical procedures in the 15 mg/kg GABA group (P value = 0.488). # > Three prior medical procedures versus < three prior medical procedures in the 30 mg/kg GABA group (P value = 0.026). Statistical tests used were the median test for two independent samples and the Kruskal-Wallis test.
4.6. Gabapentin May Reduce Postoperative Vomiting
Of all patients, 60.7% received intrathecal methotrexate chemotherapy within the procedure on the day of the experiment. Vomiting is one of the gabapentin collateral effects that could be also caused by chemotherapy. Based on the logistic regression, we found that patients who received 30 mg/kg gabapentin syrup had an odds ratio of 5.259 to not vomit (P = 0.012) (supplementary material Table 3).
5. Discussion
The optimization of analgesia in pediatric oncologic patients intends to reduce distress in young patients that experience repeated procedures. These patients are frequently sensitized to medical procedures and endure varying traumatic stress levels according to their age and environment. Gabapentin is a candidate to improve child analgesia as it is a well-established drug and easily accessible that improves analgesia in adults (13). The postoperative effects of gabapentin in children were described before (14-16). Our study aimed to assess the gabapentin effects in the perioperative period of invasive medical procedures in pediatric oncologic patients. Since gabapentin has renal elimination, it has fewer drug interactions with chemotherapeutical drugs (17).
We observed that gabapentin had no benefit in the preoperative period one hour after administration. However, gabapentin syrups reduced the time for anesthetic induction before the procedure and the amount of sevoflurane consumed. Even though sevoflurane does not produce propofol-like respiratory depression and hypotension, it induces emergence delirium in children (18). Previous studies signalized the interaction of volatile anesthetics, including sevoflurane, with α subunit of GABAA receptors (19). This can indicate a possible synergism between sevoflurane and gabapentinoids, which could explain more favorable early postoperative outcomes of gabapentin use (20).
Better results on m-YPAS at separation and anesthetic induction time points were observed probably because they were set at approximate time points and displayed the most stressful moment to the child. We consider that children below 30 points have no anxiety and our results didn’t reach this clinical situation, but the m-YPAS levels were downed with gabapentin. Alternatively, children who received gabapentin were less likely to have emergence delirium and pain postoperatively. This outcome could be most likely observed in children with less than three prior medical procedures, even though those with a higher frequency of previous lumbar punctures/biopsies may also benefit from it. Gabapentin can be strategically beneficial to oncologic patients for reducing chemotherapy-induced nausea (21) and our study indicated that gabapentin may reduce postoperative vomiting. However, further investigation is still needed to fully assess vomiting. Since we observed similar scores in the gabapentin dose groups in this study, we would indicate the use of 15 mg/kg gabapentin syrup over 30 mg/kg preoperatively in children to reduce drug intake. However, in children receiving chemotherapy, the 30 mg/kg dosage can be more effective in preventing chemotherapy-induced nausea.
5.1. Limitations
The study design was not appropriate to consistently analyze vomiting, nausea or retching in children, and thus further studies are needed.
5.2. Conclusions
Gabapentin is effective for improving anesthetic induction and reducing emergence delirium and pain following oncologic procedures. However, it exerts no clinically significant effect on preoperative anxiety. It also may reduce vomiting related to those procedures, especially when chemotherapy is used.