1. Background
Prevention and optimal postoperative bleeding have a high clinical significance in all surgeries including coronary artery bypass graft (CABG) surgery. Such management can effectively reduce the amount of bleeding and the need for blood transfusion, thus reducing the complications of blood transfusion (1). It is shown that 2% to 6% of patients undergoing CABG are again surgically treated for bleeding during and after surgery, which can be the cause of high mortality in patients (2, 3). As a result, it is very important to use interventions to reduce bleeding during or after surgery (3). Coagulopathy is one of the possible causes of excessive bleeding during and after surgery. Various factors including platelet function impairment, fibrinolysis, and coagulation factor deficiency may affect postoperative bleeding (4).
The plasma concentration of fibrinogen decreases during cardiac surgery. This change in plasma concentration may directly correlate with the amount of bleeding (5, 6). Fibrinogen is a glycoprotein and an essential component for the formation, strengthening, and enhancement of clots. Several studies have shown that fibrinogen concentration is reduced after bleeding more than the concentration of any other coagulation factor (7-9). When the coronary bypass is prolonged, the fibrinogen level is continuously lowered immediately after coronary bypass and it may reach 40% of the initial value (10). In a study, only fibrinogen and factor XIII were reduced during operation and bleeding in this condition was more related to the strength of the clot (11). A fibrinogen concentration of less than 100 mg/dL may cause severe bleeding but it is often asymptomatic. The symptoms include bleeding and disorders of wound healing. These disorders do not require treatment in most cases, but in cases requiring treatment, blood products can be used. Therapeutic action is to bring the fibrinogen level to 100 mg/dL (12). The injection of blood products in cardiac surgery has important clinical consequences including in-hospital mortality, infection and sepsis, pulmonary failure, long-term mechanical ventilation, renal dysfunction, and permanent loss of quality of life after surgery (13, 14). The normal value of the blood fibrinogen level is reported differently in various references, but on average, a concentration of 4 g/L or 200 - 450 mg/dl is normal (15, 16). More than 30 mg/kg fresh frozen plasma is needed to bring the blood fibrinogen level to 1 g/L (17). Recent studies showed a positive effect of fibrinogen after CABG, attributed to reduced bleeding and need for blood transfusions. However, previous studies have reported controversial findings (17, 18).
2. Objectives
The present study aimed to investigate the effects of prophylactic administration of fibrinogen on the rate of bleeding in patients undergoing CABG surgery to achieve more accurate clinical outcomes.
3. Methods
The current placebo-controlled, double-blind clinical trial was approved by the Institutional Ethics Committee (ethics code: IR.AJUMS.REC.1397.291) and registered at the Iranian Registry of Clinical Trials (IRCT2018090900979N1). There were 90 qualified patients undergoing elective CABG surgery from September 2018 to March 2019. However, 60 patients were consent to contribute to the study. Moreover, 24 patients did not meet the inclusion criteria. Finally, 36 patients remained in the study that were divided into two groups of fibrinogen and control (n = 18 patients in each group).
3.1. Setting and Patients
The study population included 36 patients according to the standard deviation of the blood loss volume as 150 and 268 cc in the two groups of fibrinogen and control, respectively. Concerning a power of 90% and the significance level of 0.01, the sample size was calculated as 17 patients in each group. The inclusion criteria included patients aged 50 - 70 years, candidate for CABG, with ASA physical class II-III anesthetic risk admitted to the Ahvaz Imam Khomeini Hospital from September 2018 to October 2019. The exclusion criteria included blood disorders, liver problems, pregnancy, renal disease, EF ≤ 35%, and fibrinogen level ≥ 3.5 g/L.
3.2. Randomization
Patients were randomized 1:1 to receive fibrinogen or placebo. Following a clear description of the propose and possible risks and benefits of the study, a written consent form for participation in the study was obtained from each patient. To ensure that patients and investigators were unaware of the treatment assignment before the study, a computer-generated allocation concealment process was developed before enrolling the patients. The randomization was performed after the eligibility criteria were electronically confirmed in a web-based case report form. Randomization was performed centrally without stratification and the sequence was generated by an independent statistician using a random number generator with a 1:1 allocation using random block sizes of 2, 4, and 6.
The levels of hemoglobin (HB), hematocrit (HCT), international normalized ratio (INR), prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen were assessed preoperatively. After inserting a reliable intravenous line to allow for hydration of the patients using lactated Ringer’s solution (7 mL/kg) and establishing the necessary monitoring (pulse oximetry, invasive and non-invasive blood pressure, electrocardiogram, and capnometry), the patients underwent general anesthesia with the same drugs (0.25 mg/kg midazolam, 2 µg/kg fentanyl, 1 mg/kg ketamine, and 0.5 mg/kg cisatracurium). Isoflurane 1%, 4 µg/kg/h fentanyl, 0.25 mg/kg/h midazolam, and 0.3 mg/kg/h cisatracurium were used for maintaining general anesthesia. After inducing anesthesia, we inserted a central venous catheter. For the initiation of cardiopulmonary bypass, 400 u/kg heparin was injected for all patients. Heparin dosage was adjusted base target ACT 450 - 480 second. After cutting off the bypass and administrating protamine for reversal of heparin, the patients in the case group (n = 18) were injected by 1 g of fibrinogen (CSL Behring GmbH, 35041 Marburg, Germany) dissolved in 50 cc normal saline and the control group (n = 18) received 50 mL of normal saline as placebo.
3.3. Primary Outcomes
The intraoperative hemorrhage after fibrinogen or placebo administration was calculated based on the number of bloody surgical gauzes, intra-field hemorrhage, and blood volume in milliliters collected in the bottle of the suction device under the supervision of an anesthesiologist. Injected blood, fresh frozen plasma, and platelets intraoperatively and postoperatively were also the primary outcomes. After the end of the operation, the patients were transferred to the Intensive Cardiac Care Unit in an intubated situation. The secondary outcomes including the plasma levels of fibrinogen, HB, HCT, PT, PTT, and the amount of chest tube drainage were again measured at 0, 6, 12, and 24 hours after entering the ICU.
3.4. Statistical Analysis
Statistical analysis of the data was done by SPSS version 20 (SPSS, Chicago, IL). To describe the data, the mean and standard deviation were used for quantitative variables and the frequency and percentages for qualitative variables. The data distribution was checked by the Kolmogorov-Simonov test, which showed a normal distribution. Group comparisons were made with the t-test and the chi-square test. Statistical significance was defined as a P value of < 0.05.
4. Results
During the study period from September 2018 to March 2019, there were 90 qualified patients undergoing elective CABG surgery to participate in the trial. After initial screening, 60 patients agreed to participate and provided informed consent. However, 24 patients did not meet the inclusion criteria. Finally, the remaining 36 patients were randomly divided into two groups of fibrinogen and control (n = 18 patients in each group).
There were no significant differences between the groups in baseline characteristics (P > 0.05) (Table 1).
| Variables | Fibrinogen Group | Control Group |
|---|---|---|
| Male/Female | 13 (72.2)/5 (27.8) | 12 (66.7)/6 (33.3) |
| Age, y | 59.06 ± 9.50 | 56.78 ± 8.38 |
| Height (cm) | 169.33 ± 9.04 | 161.50 ± 8.61 |
| Weight (kg) | 73.44 ± 10.07 | 74.17 ± 12.88 |
| BMI | 25.63 ± 3.20 | 28.43 ± 4.55 |
| DM | 11(61.1) | 13 (72.2) |
| HPL | 17 (94.4) | 17 (94.4) |
| HTN | 12 (66.7) | 12 (66.7) |
| Smoking | 10 (55.6) | 7 (38.9) |
| Baseline HB (g/dL) | 12.94 ± 1.88 | 13.01 ± 1.69 |
| Baseline HCT | 39.61 ± 5.27 | 40.59 ± 4.83 |
| Baseline fibrinogen (g/dL) | 3.26 ± 0.92 | 3.19 ± 0.81 |
| Baseline PT (s) | 13.11 ± 2.96 | 12.71 ± 0.36 |
| Baseline PTT (s) | 34.28 ± 16.17 | 31.22 ± 5.04 |
| Baseline PLT (μL/dL) | 268388.89 ± 74296.02 | 272666 ± 74950.96 |
| Duration of surgery (min) | 245 ± 20 | 228 ± 15 |
aValues are expressed as No. (%) or mean ± SD.
bThere was no significant difference between the groups.
There was no significant difference between the groups in the mean coagulation parameters on the first day postoperatively (Table 2).
aValues are expressed as mean ± SD.
bThere was no significant difference between the groups.
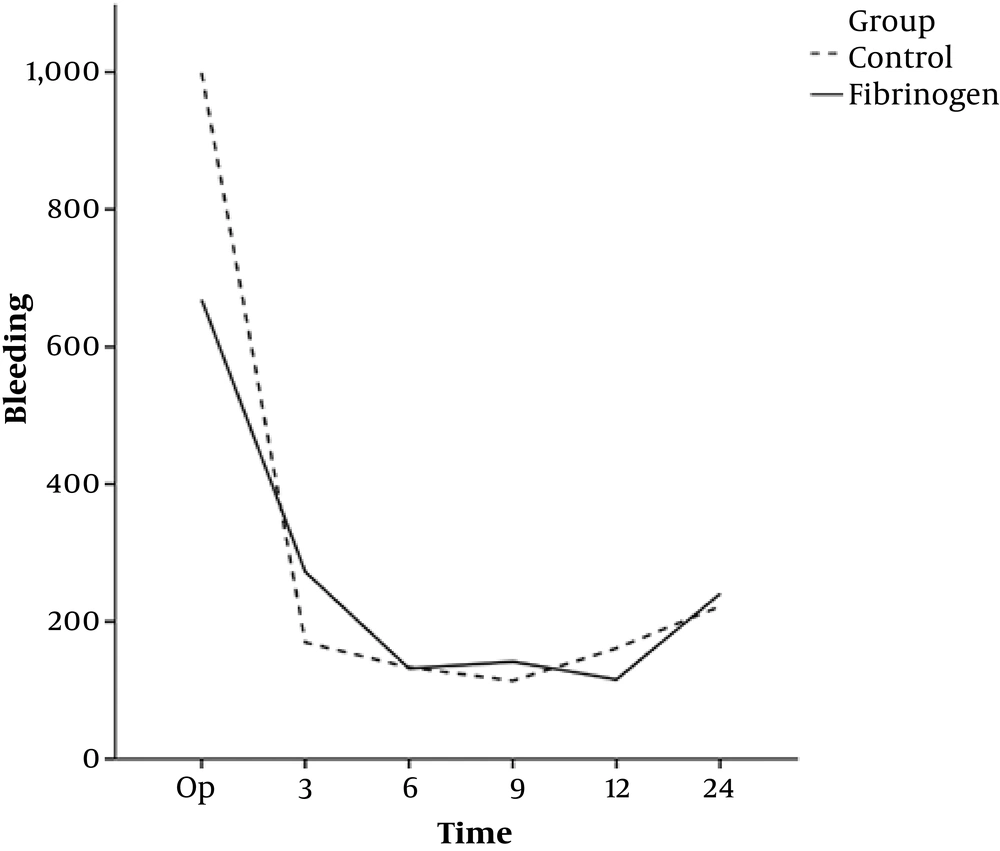
There was a significant difference between the two groups in terms of the volume of bleeding during the operation while the hemorrhage rate during the surgery was higher in the control group than in the intervention group (P ≤ 0.002). There was no significant difference in postoperative bleeding between the two groups at any of the time-points (Table 3 and Figure 1).
| Time | Fibrinogen | Control | P Value |
|---|---|---|---|
| Intraoperative bleeding (mL) | 668.33 ± 266.99 | 998.89 ± 537.76 | < 0.002b |
| Post-operative bleeding within 24 hours after operation (mL) | 901.67 ± 547.25 | 800 ± 518.20 | 0.650 |
aValues are expressed as mean ± SD.
bThere was a significant difference between the groups.
Fibrinogen injection reduced the need for blood transfusion and postoperative blood products in the intervention group when compared to the control group (P ≤ 0.005) (Table 4).
The mean arterial pressure was significantly different between the two groups at 6 hours after surgery (P = 0.072) while there was no significant difference at other time-points. The HR level of the patients was significantly lower in the case group than in the control group at 12 hours after surgery (P = 0.027). The mean duration of ICU stay (days) was significantly higher in the control group than in the fibrinogen group (3.89 ± 0.83 days versus 3.06 ± 0.42 days; P = 0.002)
5. Discussion
The present study aimed to investigate the effect of prophylactic fibrinogen injection in coronary artery bypass graft surgery to prevent intra and post-operative bleeding. We found that the intravenous administration of prophylactic fibrinogen significantly decreased the need for intraoperative blood injection and reduced the amount of bleeding during and after surgery. Most previous studies found similar findings and confirmed the effect of fibrinogen during the operation on the reduction of bleeding during and after the operation (19).
Tanaka et al. reported that prophylactic fibrinogen injection did not show differences in the amount of postoperative blood loss and the need for blood transfusion between the groups. The results showed that the replacement of primary fibrinogen could potentially reduce the incidence of infusion of platelets and red blood cells, which was consistent with our results (20).
Numerous trials have shown that the fibrinogen plasma concentration during surgery reduces the volume of bleeding and the need for blood transfusion (21, 22). Ucar et al. conducted a study on 97 patients undergoing CABG and determined that fibrinogen levels during surgery were significantly associated with bleeding 48 hours postoperatively (21). Blome et al. described a similar association between fibrinogen concentration and 24-hour bleeding in patients undergoing CABG surgery (21). In contrast, this study showed that the amount of bleeding in groups reduced only during the operation and there was no significant difference between the two groups postoperatively.
Sadeghi et al. reported no significant difference in terms of infusion of RBC during operation between the fibrinogen receiving group and the control group. There was no significant difference in bleeding during operation between the two groups. Moreover, thrombotic reactions did not occur in the two groups up to 72 hours after the operation. However, our finding did not support the finding by Sadeghi et al. so that our finding showed that bleeding during the operation was lower in the intervention group (1).
Karlsson et al. in a prospective randomized clinical trial evaluated the effects of prophylactic infusion of 2 g of fibrinogen in 20 patients undergoing CABG surgery. The patients were divided into two groups. The first group received fibrinogen and the second group received the same amount of placebo. After surgery, the fibrinogen level was higher in the intervention group than in the control group, which supports our findings. Karlsson et al. concluded that the elevated level of fibrinogen played the main role in reducing the amount of bleeding (22).
Masoumi et al. reported no significant difference in the mean hemoglobin level before and after the study. They concluded that if there is no other barrier to their use, the combination of fibrinogen and albumin has a similar effect in preventing post-surgical coagulation disorders and it is appropriate for children undergoing heart surgery, which is consistent with the results of the current study (23). Azevedo Maranhao Cardoso et al. conducted a review of randomized controlled trials in recent 10 years and concluded that the evidence confirms the beneficial effects of fibrinogen administration on reducing bleeding in cardiac surgery. It was also concluded that fibrinogen might be a good option for reducing blood transfusion in the treatment of postoperative bleeding (24), which was consistent with our results. The results of this study confirmed the results of some studies in which the use of fibrinogen was associated with the inhibition of bleeding and the reduced need for blood products.
5.1. Limitations
The limitation of this study is the lack of a follow-up of long-standing impediment resulted from operation in patients.
5.2. Conclusions
The findings of this study showed that fibrinogen plays a key role in preventing and stopping the bleeding. Accordingly, fibrinogen reduces bleeding and the necessity for transfusion in patients undergoing coronary artery bypass graft. Given the adverse outcomes of bleeding and coagulopathy in patients undergoing surgery, we concluded that the use of fibrinogen could be beneficial as a prophylactic in hemorrhagic surgery.