1. Background
Caesarean section is a common method of termination of pregnancy and there are many reasons for having a cesarean section, including delivery in older ages, reduced delivery rates, increased use of electronic birth control and so on (1). Spinal anesthesia is accepted as a safe technique for cesarean section worldwide. Since it facilitates the relationship between mother and baby and helps breastfeed the baby in the operating room, it seems more appropriate than general anesthesia (2). Spinal anesthesia has many advantages in cesarean section, including reducing the risk of aspiration of the contents of the stomach, avoiding the debilitating factors of analgesics, and the ability to stay awake (3). The proper level of anesthesia for cesarean section is the fourth thoracic nerve root (T4). A higher level of anesthesia is associated with increased risk of sympathetic paralysis and mother’s hemodynamic instability (4). Some of the disadvantages of spinal anesthesia (with topical analgesics) include shortness of postoperative analgesia, headache, damage to the pectoral nerves, nausea, urinary retention, backache, cardiac arrest, hematoma in the spinal canal with or without neurological complications, epidural abscess, and hemodynamic disorders such as hypotension and bradycardia (5).
Bupivacaine, alone or in combination with narcotics, is the most common analgesic medication used for cesarean delivery in spinal anesthesia (6). This medication causes a deep and prolonged sensory block. Using the appropriate dose of bupivacaine for spinal anesthesia can not only reduce hypotension, but it can also provide an appropriate level of spinal anesthesia for the pregnant mother. Reducing the activity of the sympathetic system after spinal anesthesia is directly associated with the decrease in the dose of bupivacaine (7-9). A dose of 8 - 10 mg is commonly used for bupivacaine (10, 11), which is associated with a high prevalence of hypotension and an increase in complications for both mother and baby (12). Bupivacaine alone can prolong the sensory and motor blockades in spinal anesthesia for lower limb surgery, compared with the combination of 10 µg epinephrine and 5 µg sufentanil (13), but the simultaneous use of dexmedetomidine and bupivacaine in spinal anesthesia for intrathecal analgesia has a longer duration of sensory and motor blockage and longer postoperative analgesia with lower side effects (14). Adding intrathecal magnesium sulphate to bupivacaine in patients under lower extremity surgeries is a safe and effective adjuvant therapeutic method for enhancing onset time of motor block (15-17).
Ropivacaine is a long-acting amide local anesthetic being alike to bupivacaine in structural and pharmacodynamics. Ropivacaine has a greater degree of separation between the motor and sensory blockade than bupivacaine and it is used to relieve epidural pain during labor or for cesarean section (3, 4, 18, 19). Adding auxiliary magnesium sulfate to ropivacaine does not increase the analgesic effect in the transversus abdominis plane block after hysterectomy (20). It is associated with a less central nervous system and other toxicities, especially cardiovascular toxicity. Several papers have reported the intrathecal use of ropivacaine for obstetric and nonobstetric patients (3-5). Many researchers have described ropivacaine as being less potent than bupivacaine (6, 19).
2. Objectives
This randomized, double-blinded study was conducted to compare the clinical profile of 10 mg hyperbaric bupivacaine 0.5% and 15 mg ropivacaine 1% in patients undergoing a cesarean section.
3. Methods
3.1. Study Design and Patients’ Recruitment
This prospective, randomized, double-blinded study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, and written informed consent was obtained from all patients. Sixty five participants scheduled for elective cesarean delivery under spinal anesthesia were randomly recruited from Imam Khomeini hospital of Ahvaz, Iran in 2018. The inclusion criteria were pregnancy (over 37 weeks and less than 42 weeks), elective cesarean section, ASA Class I - II, a weight of 65 - 95 kg, and a height of 155 - 175 cm. The exclusion criteria were patients' unwillingness to participate in the study, preterm and post-term delivery, any indication of an emergency cesarean section, ASA class higher than II, abnormalities and problems of the embryo, sensitivity to analgesics, and presence of spinal anesthesia contraindications. One group received 15 mg ropivacaine 1% (manufactured by L. Molteni & C Dei Fratelli Societa Di Eserciozio SpA, Italy) and another one hyperbaric 10 mg bupivacaine 0.5% (manufactured by AstraZeneca Sweden). The spinal anesthesia was performed in the sitting position on L4 - L5 or L3 - L4, using the Quinque spinal needle (EXEL).
3.2. Assessment and Data Collection
The sensory and motor block levels assessment was performed at 5 minutes intervals for the first 60 minutes and then recorded up to 180 minutes. The sensory block level was measured by the pinprick test. Motor block in the lower limb was assessed by a modified Bromage scale (0 = no paralysis, 1 = unable to raise extended leg, 2 = unable to flex knee, and 3 = unable to flex ankle). The patients' pain severity was assessed based on a visual analog scale (VAS) from 0 (analgesia) to 10 (severe pain). It should be noted that VAS is a self-assessment method for measuring the severity of pain. An anesthesiologist performed all assessment who was blinded to both the group assignment and the drug injected. All patients were followed up for 24 hours after surgery.
The patients’ blood pressure was measured and recorded every five minutes in the first 30 minutes and after that, was recorded every 15 minutes. If hypotension decreased below 90 mmHg or 25% lower than the initial blood pressure, 5 mg intravenous ephedrine was injected. If any signs of bradycardia were observed (heart rate less than 60 ppm), the patients were treated with 1 mg atropine intravenously. Hemodynamic parameters including systolic and diastolic blood pressure and baseline heart rate were recorded before the injection.
3.3. Statistical Analysis
The data were analyzed by SPSS (version 22). Repeated measurement analysis and independent t-test were performed in this research.
4. Results
Sixty five people with a mean age of 28.55 ± 5.5 years (18 - 40 years) participated in this study. There was no significant difference between the two groups in terms of age, weight, height, BMI, and gestational age (P < 0.05) (Table 1).
| Group | Mean ± SD | Test Statistic (P Value) |
|---|---|---|
| Age, y | -1.293 (0.201) | |
| Ropivacaine | 27.63 ± 5.04 | |
| Bupivacaine | 29.43 ± 5.96 | |
| Gestational age, wk | -1.258 (0.215) | |
| Ropivacaine | 37.75 ± 2.97 | |
| Bupivacaine | 38.48 ± 0.96 | |
| Height | 0.583 (0.562) | |
| Ropivacaine | 165.69 ± 7.24 | |
| Bupivacaine | 164.53 ± 8.56 | |
| Weight, kg | -0.158 (0.875) | |
| Ropivacaine | 76.94 ± 10.78 | |
| Bupivacaine | 77.34 ± 9.69 | |
| Duration of surgery, min | 1.248 (0.210) | |
| Ropivacaine | 9.02 ± 51.61 | |
| Bupivacaine | 8.41 ± 50.12 | |
| BMI, kg/cm2 | -2.024 (0.05) | |
| Ropivacaine | 25.23 ± 8.97 | |
| Bupivacaine | 28.61 ± 3.40 |
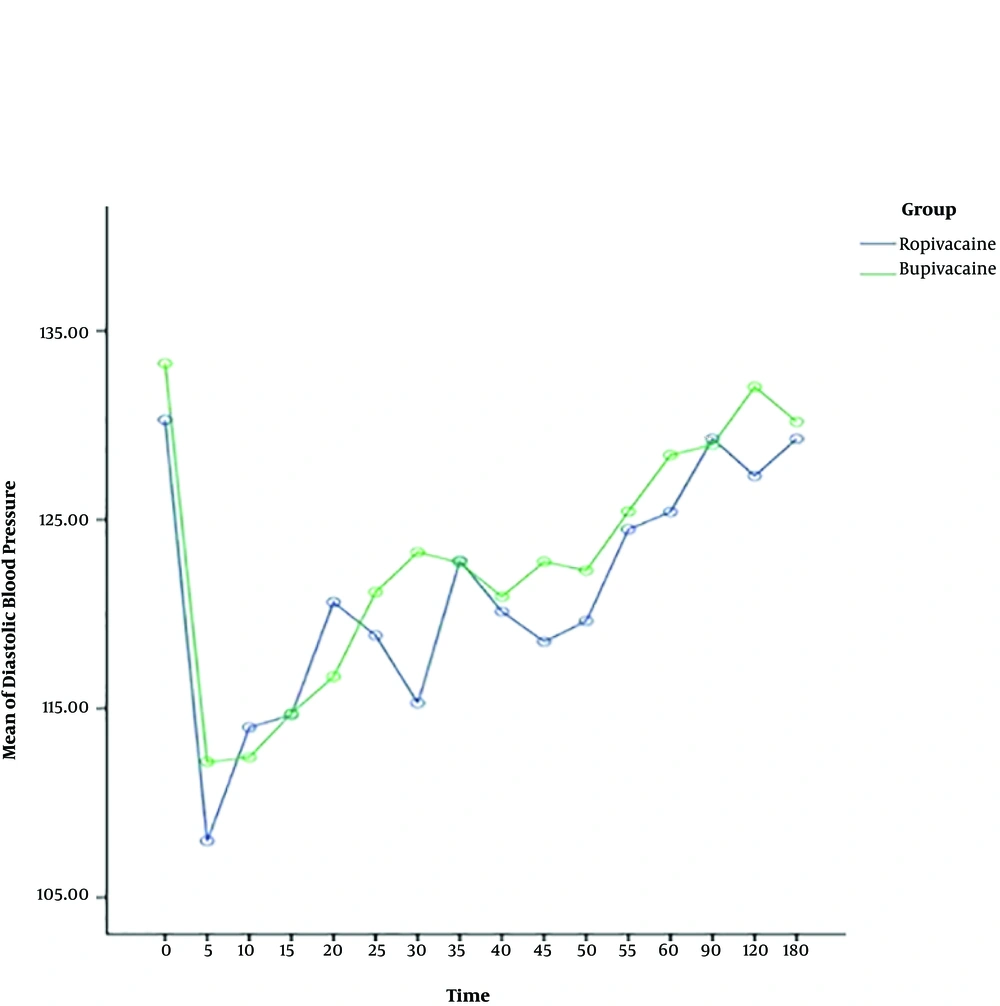
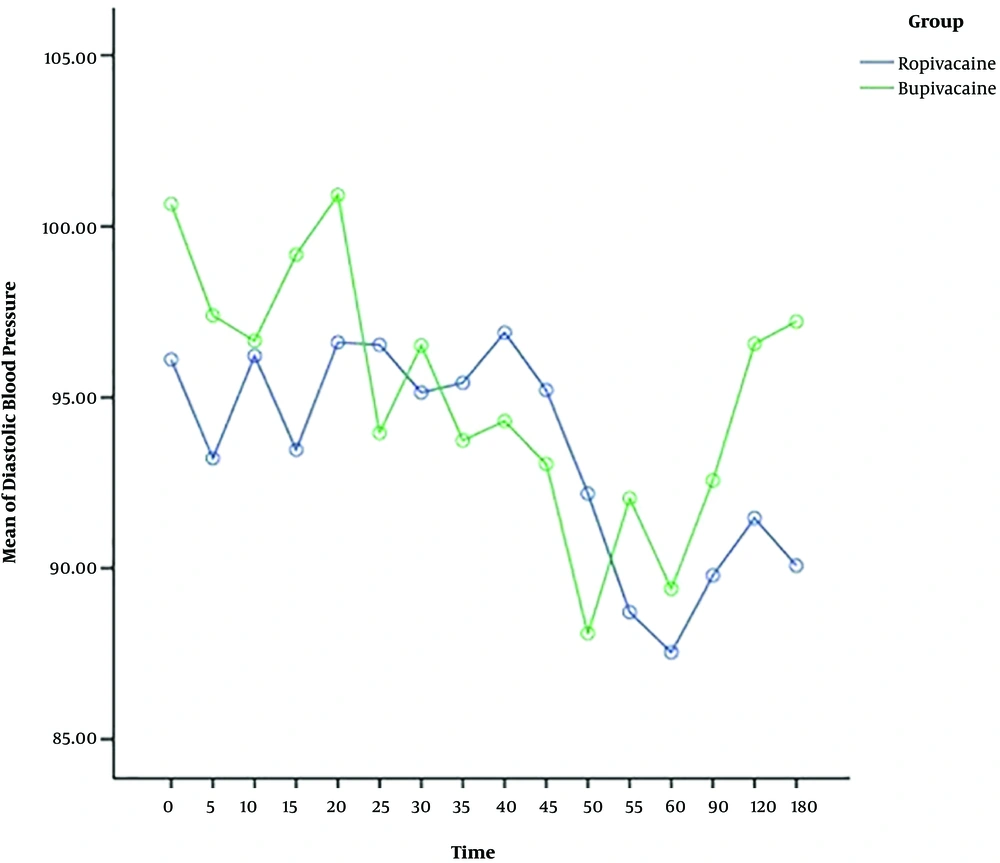
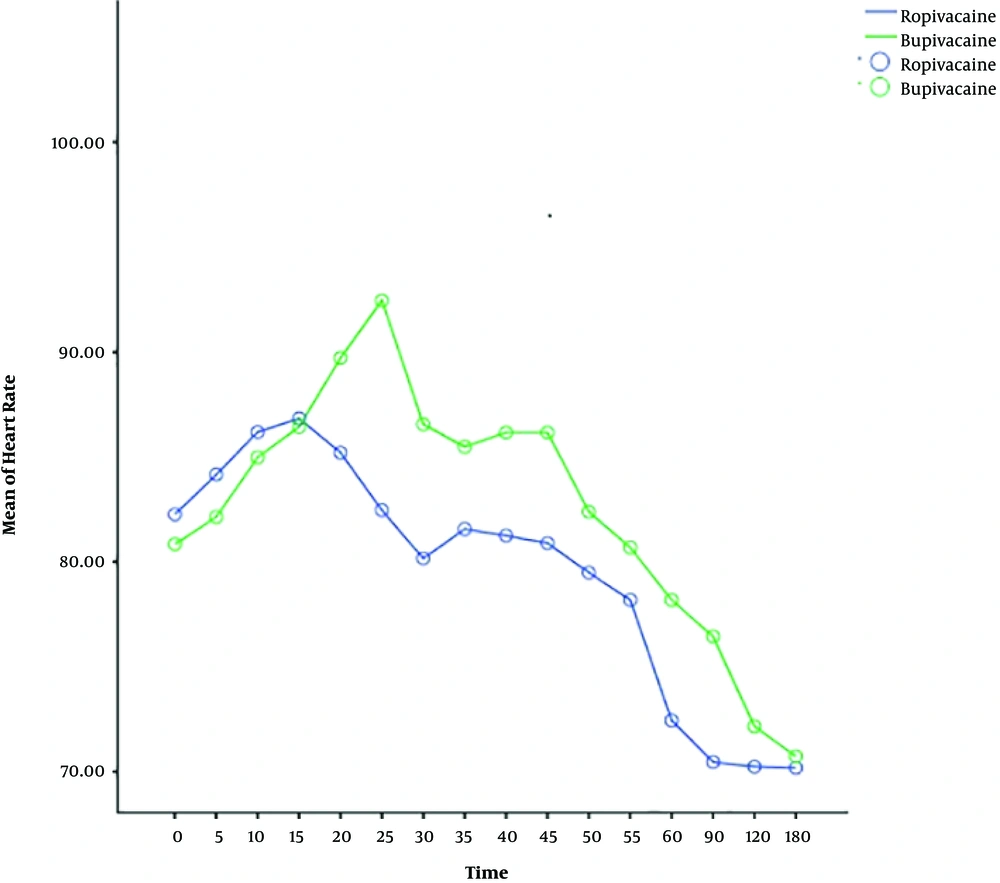
The heart rate, systolic and diastolic blood pressure change significantly during time, and also the trend of changes is almost similar in both ropivacaine and bupivacaine groups. The results show that there is no difference between two groups in terms of systolic and diastolic blood pressure, but the heart rate of patients for bupivacaine group is significantly higher than ropivacaine group, around more than 75 percent of times (Table 2) (Figures 1 - 3).
| Variable, Source | Mean Square | F | P Value |
|---|---|---|---|
| Systolic blood pressure | |||
| Time (within group) | 2356.81 | 10.48 | < 0.001 |
| Time × group | 118.57 | 0.53 | 0.927 |
| Error | 224.94 | ||
| Group (between group) | 766.871 | 0.995 | 0.323 |
| Diastolic blood pressure | |||
| Time (within group) | 431.96 | 2.45 | 0.002 |
| Time × group | 152.01 | 0.86 | 0.609 |
| Error | 176.511 | ||
| Group (between group) | 603.197 | 0.596 | 0.444 |
| Heart rate | |||
| Time (within group) | 1942.176 | 83.716 | < 0.001 |
| Time × group | 30.622 | 1.32 | 0.130 |
| Error | 23.199 | ||
| Group (between group) | 2598.918 | 113.282 | < 0.001 |
The onset of motor and sensory block in the bupivacaine group was significantly faster than the ropivacaine group (P < 0.001). The complete sensory and motor blockage in the ropivacaine group was significantly faster than the bupivacaine group (P < 0.001) (Table 3).
| Variable, Group | Mean ± SD | Test Statistic (P Value) |
|---|---|---|
| Onset of sensory block | -6.051 (< 0.001) | |
| Ropivacaine | 2.32 ± 0.9 | |
| Bupivacaine | 1.28 ± 0.4 | |
| Time to complete sensory block | 7.258 (< 0.001) | |
| Ropivacaine | 132.5 ± 21.6 | |
| Bupivacaine | 175.8 ± 26.2 | |
| Onset of motor block | -7.798 (< 0.001) | |
| Ropivacaine | 2.86 ± 0.82 | |
| Bupivacaine | 1.63 ± 0.38 | |
| Time to complete motor block | 8.340 (< 0.001) | |
| Ropivacaine | 124.8 ± 20.2 |
There was no significant difference between two groups in terms of pain severity at different times (P > 0.05) (Table 4). No neurological changes, backache, headache occurred within 24 hours of discharge in either group.
| Variable, Group | Mean ± SD | Test Statistic (P Value) |
|---|---|---|
| Skin profile | 1.729 (0.088) | |
| Ropivacaine | 1.95 ± 0.9 | |
| Bupivacaine | 1.76 ± 0.6 | |
| Uterus profile | -1.90 (0.061) | |
| Ropivacaine | 1.87 ± 0.4 | |
| Bupivacaine | 1.60 ± 0.7 | |
| End of surgery | 1.152 (0.253) | |
| Ropivacaine | 1.17 ± 0.7 | |
| Bupivacaine | 1.37 ± 0.7 | |
| Completing the recovery | 0.492 (0.623) | |
| Ropivacaine | 1.67 ± 0.6 | |
| Bupivacaine | 1.75 ± 0.7 |
5. Discussion
In the cesarean section, anesthetic drugs may impact on the pregnant woman and fetus (21). Ropivacaine is a L-amide anesthetic that is similar to bupivacaine in structural and pharmacodynamics. Ropivacaine has the advantage of separated sensory and motor block, with less toxicity to the cardiovascular system and central nervous system (22-24). Some studies have shown that ropivacaine is more effective in sensory and motor block being inferior to the lidocaine but superior to the bupivacaine (25).
This study aimed at investigating the clinical efficacy and safety of spinal anesthesia of ropivacaine 1% and bupivacaine 0.5% for elective cesarean delivery. We found that 15 mg ropivacaine 1% produced a parallel and effective clinical profile with shorter duration of sensory and motor block, compared with 10 mg hyperbaric bupivacaine 0.5% for elective cesarean section, although the onset time of sensory and motor block of ropivacaine was significantly longer than that bupivacaine. Wang et al. (26) demonstrated that ropivacaine is more recommended due to little effect on the hemodynamics, shorter duration of sensory block and motor block, and lower incidence rate of side effects, which are beneficial to the recovery and also provide safety to the patients while either a low or high dose of ropivacaine and bupivacaine results in the discomfort (27). Chung et al. showed that 18 mg of 0.5% hyperbaric ropivacaine provided similar and effective spinal anesthesia with shorter duration of sensory and motor block, compared with 12 mg of 0.5% hyperbaric bupivacaine for cesarean delivery (28). In a meta-analysis study in 2016 the findings indicated that intrathecal ropivacaine reduces the duration of motor block and it has a similar onset of sensory block and no difference in the incidence of maternal hypotension (29). Hence, ropivacaine is more conductive for cesarean section, with higher satisfaction and more rapid recovery in the motor and it can be an alternative to bupivacaine for cesarean section (26, 30).
The present study displayed that systolic and diastolic blood pressure changes during the time but there is no significant difference between ropivacaine and bupivacaine groups in this issue. The heart rate of patients for the bupivacaine group is significantly higher than the ropivacaine group. Since bupivacaine provides potent cardiotoxicity, it may increase heart rate and generate discomfort for some pregnant women (24, 31). But, ropivacaine has a lower lipid solubility than bupivacaine which produces a less toxic effect on the cardiovascular system and heart (31).
5.1. Conclusions
In conclusion, 15 mg ropivacaine 1% produced a parallel and effective clinical profile with shorter duration of sensory and motor block, compared with 10 mg hyperbaric bupivacaine for elective cesarean section, although the onset time of sensory and motor blockage of ropivacaine was significantly longer than that of bupivacaine. There is no difference between groups in terms of systolic and diastolic blood pressure, but the heart rate of patients in the bupivacaine group is significantly higher than the ropivacaine group. Therefore, ropivacaine can be an alternative to bupivacaine for cesarean section.