1. Background
Heart surgery is usually accompanied with blood loss and hemorrhagic syndrome (1). Bleeding after coronary artery bypass graft surgery in use of cardiopulmonary bypass pump is reported around 25% - 50%. It can cause morbidity and mortality in cardiac surgery. The main reason of bleeding in cardiac surgery is reaction between blood and inner surface of the cardiopulmonary bypass pump, which affects the platelet activity, increases fibrinolytic reactions and coagulation disorders (2). The post-operative bleeding is the result of a complex reflection of various hemostatic defects, including coagulation factor deficiency, inadequate reversal heparinization, increased fibrinolytic activity, and platelet deficiency (2, 3).
In recent years, one of the most important concerns in this practice has been the application of measures to manage and control bleeding during and after surgery (4). Despite many developments in blood preservation techniques, blood transfusion in heart surgery is still used extensively (5, 6). The use of blood products has crucial clinical consequences such as increasing transmission rate of infectious diseases and viruses, suppressing the immune system and postoperative infections, increasing hospital mortality, long-term mechanical ventilation, and a permanent loss of post-surgical quality of life (7, 8). Murphy et al. reported that blood transfusion in heart surgery patients is highly associated with infection and post-operative ischemic disease, prolonging hospital stay, increasing morbidity and mortality and hospital costs (9). In addition, blood is a limited and costly resource, and some people are not eager to receive blood (10, 11). Cardiopulmonary bypass surgery also affects the inflammatory response in addition to the hemorrhage. This inflammatory response may result in postoperative complications such as cardiac, renal, neurological, liver dysfunction, respiratory failure, bleeding disorders, and ultimately multiple organ failure (12, 13).
Many studies have been conducted to control bleeding and inflammation with anti-fibrinolytic drugs and vitamin supplements such as vitamin C. Anti-fibrinolytic tranexamic acid is a combination derived from lysine amino acids (4-amino ethyl cyclohexane carboxylic acid), which by blocking the function of plasmin, prevents blood clotlysis and fibrinolysis (14, 15). Antioxidant vitamins such as vitamin C (ascorbic acid, ascorbate) are used to control bleeding and inflammation in various procedures, heart surgery and AF (atrial fibrillation) arrhythmia control after heart surgery (16-24).
Atrial fibrillation is a common disorder after cardiac surgery that is seen in 15% - 50% of patients (25) and makes the cardiac mortality and morbidity threefold and the risk of embolism fourfold. It prolongs the staying time of patient in the hospital and has a great negative effect on the quality of the patient’s life (26, 27).
This vitamin blocks free radicals in the body and prevents their damage to the body and plays an important role in enhancing the immune system, production of collagen, better absorption of iron in the body, eliminating anemia, strengthening the capillary wall, and helping blood coagulation. Vitamin C deficiency causes atherosclerosis, and its deficiency in patients who suffer from heart disease causes stiffness of the veins and pain (16-24).
2. Objectives
Considering different views on the use of these drugs, this study was designed to evaluate and compare the effect of intravenous tranexamic acid (TA) and combined intravenous tranexamic acid with vitamin C (TXC) on the volume of drainage (bleeding) and AF arrhythmia in patients undergoing cardiac bypass surgery.
3. Methods
This study is a double blind randomized clinical trial. After approval by the Ethics Committee of Golestan University of medical science this study was conducted between 2016 and 2018 in Shafa Hospital of Gorgan.
Total of 120 coronary artery bypass graft surgery (CABG) candidates were participated in this study (Figure 1).
All patients after giving the necessary explanations and obtaining written consent were enrolled in the study. For random allocation, computer numbers were utilized. Patients were not aware of the type of medication. A nurse anesthetist who assessed the patients had no information on the type of intervention.
Inclusion criteria: The age group of 30 - 70 years, EF > 30%, hemoglobin > 10 g/dL, written and oral informed consent of the patient, first cardiac surgery, BMI < 24, not have drug sensitivity; no history of seizure and coagulation disorders.
Exclusion criteria: Patients with chronic disease such as rheumatoid arthritis, ischemic heart disease, malignancy, history of any previous thromboembolic episodes, hemoglobin < 8 g/dL were excluded from the study.
3.1. Procedure
Patients were randomly assigned to treatments in two groups: group A (TA) and group B (TXC). All patients were administered by the same anesthetic and surgical team in the same surgical operations. One day before surgery each patient was examined by a cardiac anesthesia fellowship and their medical histories and tests were checked and recorded.
In both groups, 50 mg/kg tranexamic acid (Tranexip®, Caspian Tamin Pharmaceutical Co. Tehran-Iran) was administered intravenously directly before sternotomy. In group B, half an hour before surgery, 2 g of vitamin C and 100 mL 0.9% saline were injected (Darupakhsh Pharmaceutical Co. Tehran-Iran). Subsequently, during 4 days after surgery, 1000 mg of vitamin C and 100 cc 0.9% saline was infused every day.
In this study, the amount of drainage (bleeding) during and after surgery determined by weighing sponges and surgical gauzes-measuring bottle of suction and chest tube drainage and AF arrhythmia was recorded up to 24 hours. Hemodynamic parameters (heart rate, systolic, diastolic and mean arterial blood pressure (MABP), arterial oxygen saturation, CVP and ABG, urinary output, blood glucose levels, temperature, blood electrolytes, fluid intake control, Hb, HCT, ACT, PT, PTT were monitored and recorded in all patients during and after surgery. The surgical and anesthetic team was the same in all patients and the anesthetic method was as follows.
3.1.1. Anesthesia Induction
Intravenous injection of 10 µg/kg fentanyl, 0.5 mg/kg midazolam, 5 mg/kg Nesdonal (Sodium thiopental), 0.5 mg/kg atracurium relaxant, and 1.5 mg/kg Succinylcholine.
3.1.2. Anesthetic Maintenance
All patients received a combination of 10mg midazolam + 100 mg atracurium + 1 mg fentanyl and inhaled iso-fluorane with 1.5 MAC. The patient’s temperature ranged from 28 to 34°C during the pump. The patient received 300 - 400 units/kg heparin 2 minutes before going on the pump for a period of 2 hours, and by prolonging the pump time, the same amount of heparin was repeated, and eventually, patients received protamine at 1/3 dose of heparin. Meanwhile, hemoglobin levels below 7 g/dL, PT and PTT abnormalities, hematocrit less than 20, and platelets less than 100,000 and transfusions of blood products were controlled.
3.1.3 Postoperative Patient Control in the Cardiac ICU
1. Hemodynamic control (heart rate; systolic, diastolic and median arterial blood pressure (MABP), CVP; arterial oxygen saturation) with SAADAT monitoring every 15 minutes until stabilizing the condition.
2. Patient’s ABG control (4 times before changing each ventilation mode).
3. Controlling Hb, Hct levels after surgery, chest drainage (bleeding), urinary output, and blood glucose levels.
3.2. Data Analysis
The analysis of this study was performed using statistical software SPSS15. Normality of variables was evaluated with Kolmogorov-Smirnov test. To compare the mean of quantitative variables in two groups, if the data was normal, independent t-test and otherwise Mann-Whitney test was used. Relationship between qualitative variables was analyzed by chi-squared test, and otherwise Fisher’s exact test. The significance level was considered as P < 0.05.
4. Results
This study was performed on 120 patients undergoing cardiac surgery. Patients were divided into two groups: TA (58) and TXC (62). The mean and standard deviation (mean ± SD) of the age in the TA group and in the TXC group were 62.19 ± 9.23 and 60.10 ± 9.92, respectively.
Data analysis demonstrated no significant difference between the two groups in mean age, weight, and BMI (Table 1).
| Characteristic | Group TA (N = 58) | Group TXC (N = 62) | P Value t Test |
|---|---|---|---|
| Age, y | 62.19 ± 9.23 | 60.10 ± 9.92 | 0.23 |
| Body mass index, kg/m2 | 27.38 ± 4.59 | 29.73 ± 16.46 | 0.65 |
| Weight | 74.52 ± 13.48 | 75.26 ± 14.91 | 0.74 |
aValues are expressed as mean ± SD.
Chi-square test demonstrated that the two groups were homogeneous in terms of gender, occupation, history of heart disease, hypertension, diabetes, aspirin use, narcotics, and smoking. Also, Fisher’s exact test between the two groups did not show a significant difference in the history of heart disease in the family (P > 0.05). In the present study, 65% (78 patients) had a history of heart disease, 45.8% (55) had a family history of diabetes, and 80% (96 patients) had a history of hypertension (Table 2).
| Variables | Group TA | Group TXC | P Value |
|---|---|---|---|
| Gender | 0.92b | ||
| Male | 37 (63.8) | 39 (62.9) | |
| Female | 21 (36.2) | 23 (37.1) | |
| Occupation (job) | 0.83b | ||
| Employee | 9 (15.5) | 12 (19.4) | |
| Free | 9 (15.5) | 8 (12.9) | |
| Farmer | 7 (12.1) | 5 (8.1) | |
| Housewife | 19 (32.8) | 18 (29.) | |
| Retired | 14 (24.1) | 19 (30.6) | |
| History of heart disease | 0.20b | ||
| Yes | 41 (70.7) | 37 (59.70 | |
| No | 17 (29.3) | 25 (40.3) | |
| History of heart disease in the family | 0.50c | ||
| Yes | 20 (34.5) | 25 (40.3) | |
| No | 38 (65.5) | 37 (59.7) | |
| Aspirin | 0.10b | ||
| Yes | 42 (72.4) | 36 (58.1) | |
| No | 16 (27.6) | 26 (41.9) | |
| Blood pressure | 0.23b | ||
| Yes | 49 (84.5) | 47 (75.8) | |
| No | 9 (15.5) | 15 (24.2) | |
| Opiate use | 0.12b | ||
| Yes | 14 (24.1) | 23 (37.1) | |
| No | 44 (75.9) | 39 (62.9) | |
| Cigarette use | 0.97b | ||
| Yes | 12 (20.7) | 13 (21.0) | |
| No | 46 (79.3) | 49 (79) | |
| Diabetes | 0.60b | ||
| Yes | 28 (48.3) | 27 (43.5) | |
| No | 30 (51.7) | 35 (56.5) |
aValues are expressed as No. (%).
bP value was determined using chi-square test.
cP value was determined using the Fisher exact test.
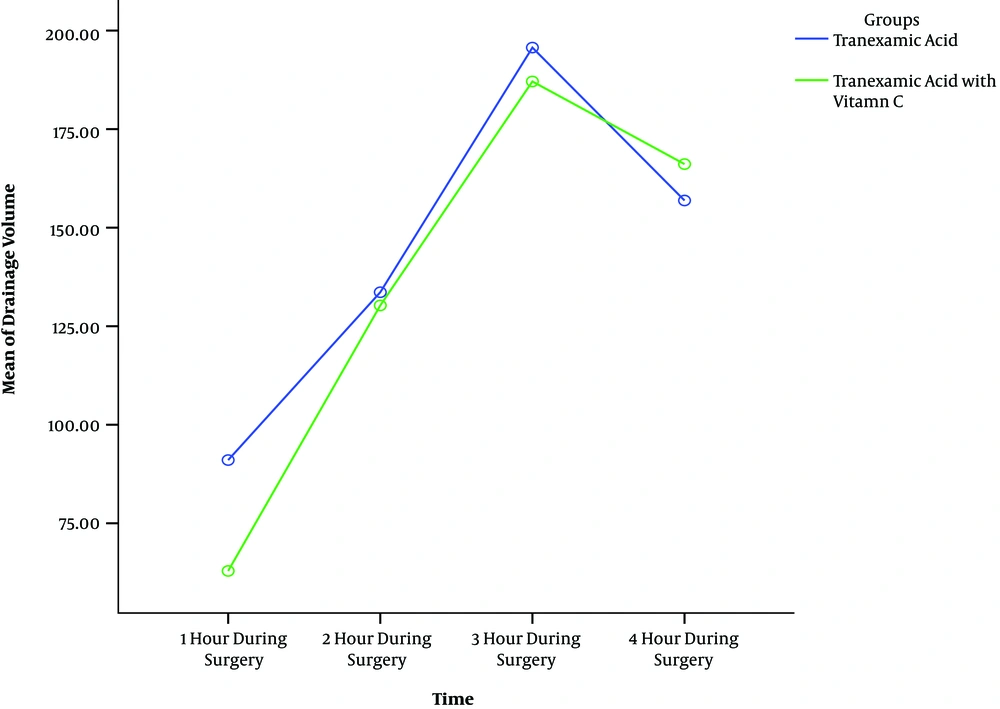
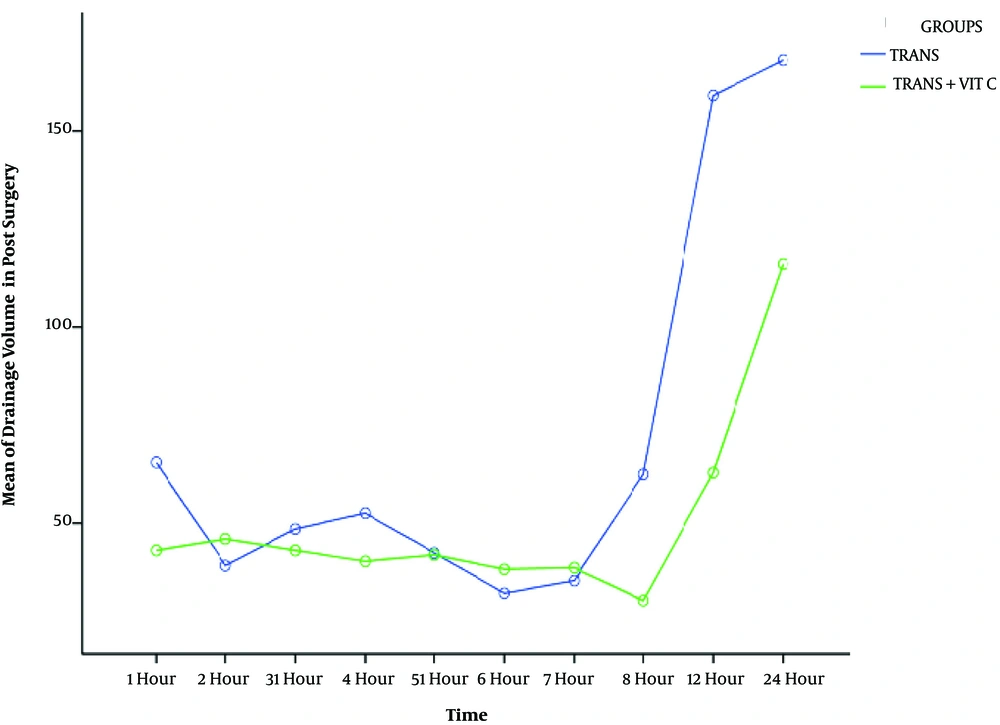
In this study group, TXC had less bleeding during operation and in the early hours of post-operation (Table 3 and Figures 2 and 3).
| Parameter | Category | EST | Std. Error | P Value |
|---|---|---|---|---|
| Intercept | - | 44.97 | 5.98 | < 0.001 |
| Group | Control | 34.41 | 8.48 | < 0.001 |
| Intervention | Reference category | |||
| Time | - | 36.65 | 2.60 | < 0.001 |
| Interaction | Control | -10.68 | 3.72 | 0.004 |
| Intervention | Reference category | |||
For group variable, the estimate of 34.41 mL tells us that patients in TA group had mean drainage of 34.41 mL more than patients in TXC group (P < 0.001).
The estimate of about 36.65 mL for time variable means that every hour during surgery, patients experienced a mean drainage of 36.65 mL units more than the previous time (P < 0.001) (Table 3).
The significance estimate for the interaction parameter means that two groups had significant difference during the under study period regarding mean drainage (P = 0.004) (Table 3).
4.1. The AF Arrhythmia Condition
Chi-square test showed that arrhythmia AF condition was the same in the two groups during the 14 times of assessment (four hours during operation and ten hours up to 24 hours after the operation) and the arrhythmia in the two groups was less than 5%.
5. Discussion
The aim of this randomized clinical trial study was to evaluate the efficacy of tranexamic acid and tranexamic acid combined with vitamin C on drainage volume (blood loss) and AF arrhythmia in patients undergoing cardiac bypass surgery.
The result of this study demonstrated that there was a significant difference in the bleeding control of the TXC group during the first hours of surgery and the two groups were similar in AF arrhythmia in the 24 hours after surgery. In both groups, regarding this arrhythmia there was no statistically significant difference, although it was clinically superior in vitamin C group.
Results of other studies reported that patients undergoing surgery under the CPB (cardiopulmonary bypass) heart pump are at increased risk for vitamin C deficiency. Rodemeister et al. reported that during the heart surgery due to increase in MDA (malondialdehyde) level, a marked decrease in plasma levels of vitamin C occurred and no increase in its concentration occurred until one week after surgery (28).
Oktar et al. reported levels of vitamin in the blood plasma being much lower pre, during and on the next day after CABG in patients without supplementation with vitamin C in CABG (29).
In this study, we observed a positive effect of vitamin C in the first hours after the injection in control of bleeding, but at a later time there was no significant effect, and this could be due to the loss of plasma levels of the drug.
Ker et al. reported that TA has significant impact on reduction in hemoglobin loss, the mean number of consumed blood products, and reoperations after cardiac surgery compared with placebo (30). Alshryda in a systematic review and meta-analysis study demonstrated that tranexamic acid is effective in reducing postoperative hemorrhage in cardiac surgery and mortality rate in patients who suffer from upper gastrointestinal problems. In this study patients’ bleeding rate decreased more than 50% (31). The evidence reported that vitamin C, due to its anti-inflammatory properties, is an important antioxidant in reducing drainage volume (bleeding), electrical regeneration and atrial fibrillation, postoperative pain, pulmonary complications, and dysfunction of other organs after heart surgery (32). Studies demonstrated that there is a strong correlation between the use of vitamin C and the reduction of the incidence of AF after surgery, which may be due to a reduction in the inflammation of oxidative factors (17, 23).
Vitamin C, because of its anti-inflammatory properties, effectively reduces activity of free radicals and decreases membrane lipid peroxidation. It can reduce indicators of cardiac damage, and improves hemodynamic condition. This property of vitamin C affects the amount of drainage (bleeding) and the duration of hospitalization and staying in ICU (33, 34). Several factors such as smoking, aspirin, alcohol, obesity and aging may affect the complex network of mechanisms regulating vitamin C and its transmission and its physiological effects also considering that parameters of oxidative stress increase significantly during and after cardiac bypass surgery and it is the cause of damage to tissues (35, 36).
5.1. Arrhythmia
In both groups, AF arrhythmia was less than 5%, and there was no statistically significant difference, although it was clinically superior in vitamin C group. Studies reported that vitamin C plays a very important role in controlling arrhythmia and cardiovascular events, regulating and controlling blood pressure and reducing the incidence of POAF (postoperative atrial fibrillation) and is safe and affordable, this effect can be due to reduced inflammatory and oxidative factors (21, 37-39).
A meta-analysis study by Harling et al. demonstrated that vitamin C supplementation is effective in controlling AF arrhythmia and other arrhythmias (40). Also Carnes et al. examined 43 patients undergoing heart surgery with vitamin C before and up to 5 days postoperatively and reported that the prevalence of POAF in patients treated with ascorbate was 16.3% compared to 34.9% in the control group (41).
Papoulidis et al. with research on 85 patients in each group demonstrated significant reduction in the incidence of postoperative AF in patients who received vitamin C (44.7% vs. 61.2%). Nevertheless studies by Bjordahl et al. and Antonic et al. demonstrated that ascorbic acid supplementation does not have effectiveness in reducing the incidence of postoperative atrial fibrillation on CABG patients (42, 43).
The contradictory results of the various studies should be considered that the pathogenesis of POAF is a multi-factorial condition, an increase in sympathetic withdrawal associated with hypovolemia, anemia, hypoxia or pain in surgical patients, pericardial manipulation, and local inflammation during chest and heart surgery may be associated with surgery. Metabolic and electrolyte problems and blood transfusions and the nutritional status of different populations can vary and also affect the incidence of POAF (42, 44). The Johnston study found that vitamin C intake was inadequate in 20% - 30% of the American population (43, 45).
5.2. Limitations
In this study we did not measure the level of vitamin C in different times during and after surgery because it was not evaluated in the plasma.
5.3. Conclusions
According to results of this study, it can be concluded that the use of tranexamic acid with vitamin C supplementation has a positive effect on reducing the amount of drainage (bleeding) and the incidence of AF arrhythmia after surgery, and it is suggested that by examining the plasma level of vitamin C and its stabilization, the effect of this supplement in cardiac surgery and other operations be investigated. We suggest more research on the effects of ascorbic acid on CABG operations.