1. Background
The management of pain after thoracotomy and video-assisted thoracoscopic surgery (VATS) is important given that these procedures are considered to be amongst the most painful of operations (1). Optimising analgesia in this context may avoid significant morbidity including atelectasis, intra-pulmonary shunting, hypoxaemia, infection, delayed mobilisation, longer hospital stay, and chronic pain (2). Surgically sited extrapleural local anaesthetic (LA) blocks and catheters can safely provide effective analgesia for patients for several days following thoracotomy and VATS (3). Extrapleural LA delivery via indwelling catheter permits analgesic neural blockade beyond the duration of a single dose. Traditionally this has been done by continuous infusion (CI), however doubts remain regarding the maximal efficacy of CI as the extrapleural space is large, potentially restricting the range of spinal nerves covered by LA (4). This is of particular concern as infusion rates are restricted by maximum recommended daily LA doses to 28 mg/h ropivacaine (14 mL/h ropivacaine 0.2%) (5). In a similar manner to the concept of the use of ‘rescue boluses’ of larger doses of LA to re-establish analgesia via epidural catheters, programmed intermittent boluses (PIB) theoretically offers a potential advantage over CI by achieving a larger range of spinal nerves covered by LA in the extrapleural space.
PIB has achieved superior analgesia over CI via the epidural (6), femoral (7), adductor canal (8) and popliteal (9) routes. Prospective randomised studies conducted specifically in thoracic surgery however suggest no difference in efficacy (10), or inferiority, of PIB to CI. These studies have not explored the efficacy of higher PIB frequency than 6-hourly with a low background infusion. In order to explore the potential benefit of high frequency, low background rate extrapleural PIB LA, from 2016 our tertiary centre introduced dedicated regional analgesia delivery devices that were capable of automated intermittent bolusing with a continuous background infusion rate, and our acute pain service formalised a standard PIB protocol that could be activated according to clinician discretion after a regional LA catheter was placed. We hypothesized that high frequency, low background infusion extrapleural PIB could achieve superior patient analgesia and reduced oral morphine equivalent daily dosage (OMEDD) requirements for up to 3 days after thoracic surgery when compared against CI and adjusted for patient factors, daily LA doses, surgery type, and adjunctive analgesic techniques.
2. Methods
Following institutional ethics review (LNR/17/Austin/466), we conducted a retrospective, single-centred, observational study to assess adult patients who received LA via a surgically sited extrapleural paravertebral catheter following VATS or thoracotomy at the Austin Hospital from September 2016 to November 2017. The Austin Hospital is a tertiary level university teaching hospital in Melbourne, Australia, with a dedicated high-volume thoracic surgical service.
The extrapleural paravertebral catheters were all placed intraoperatively under direct vision by the surgeon and were loaded with a bolus of 30 mL 0.5% ropivacaine. Our default institutional protocols for analgesia after thoracic surgery are (1) postoperative extrapleural LA continuous infusion of ropivacaine 0.2% at 10 mL/h (range 0 - 14 mL/h); or background continuous infusion of ropivacaine 0.2% at 5 mL/h (range 0 - 5 mL/h) with 2 hourly programmed boluses of 6 mL (range 0 - 10 mL); and (2) intravenous opioid delivered by patient controlled analgesia (PCA) of one of: morphine 1 mg demand dose/5 min; fentanyl 20 mcg demand dose/5 min; or oxycodone 1mg demand dose/5 min. The initial choice of either LA regimen and PCA opioid type was at the discretion of the treating anaesthetist. Subsequent changes to LA infusion and opioid orders were made by the acute pain service on daily patient review to achieve postoperative recovery goals e.g. effective deep breath and cough, ability to sit out of bed, and mobilisation, rather than NRS-11 scores alone. LA bolus doses, but not frequency (if PIB)/or infusion rate (if CI) were increased if the above recovery goals were not met; both dose and frequency/infusion rate were reduced if patients reported early symptoms of local anaesthetic systemic toxicity (LAST), e.g. perioral numbness or tingling. Regular and/or breakthrough adjunctive analgesics (paracetamol; non-steroidal anti-inflammatory analgesics; tramadol) were prescribed if no patient contraindications were present. If patients did not meet the above recovery goals our extrapleural prescription protocol suggested 2 rescue boluses of 12 mL 0.2% extrapleural ropivacaine at least 20 mins apart as needed. Patients were instructed to use their PCA to achieve satisfactory analgesia, and to achieve deep breathing and coughing four times a day without prohibitive pain.
Patients were included for analysis if they underwent thoracotomy or VATS, and had a surgically sited extrapleural catheter for delivery of LA. Patients were excluded if they had thoracotomy or VATS for minimally invasive cardiac surgery, oesophagectomy, or any combined abdominal and thoracic procedure. Patients were also excluded if they received intrathecal morphine or were opioid-tolerant pre-operatively, defined as greater than 50 mg of oral morphine equivalent daily doses (OMEDDs) (11). In addition, patients were excluded if they had already been included earlier in the study period, or if they underwent multiple procedures during the one hospital admission.
Patient’s demographic information, NRS-11 pain assessments, total LA infusion and bolus doses, and length of hospital stay were retrieved from our institution’s medical records. Post-operative opiate use via oral and parenteral administration, including patient-controlled analgesia, was recorded and converted to OMEDDs as per Australian and New Zealand College of Anaesthetists (ANZCA) and Faculty of Pain Medicine (FPM) official documents (12). NRS-11 pain ratings were obtained by ward nursing staff with patients asked to provide a verbal rating on the severity of their pain from zero to ten, where zero is no pain and ten is the worst pain imaginable. The maximum and minimum pain scores in each 24-hour postoperative period for 3 postoperative days were recorded. We selected these pain outcomes to represent pain at times of greatest activity (e.g. physiotherapy, deep breathing and coughing, transfers from bed) versus those at times of quiet rest (representing times of best possible analgesia). “Twenty-four-hour period” was defined as 0800 - 0800 hrs, with day 0 defined as operation start time to 0800 h the following day. Adverse outcomes, including the activation of the intensive care unit’s medical emergency team (MET), symptoms of local anaesthetic systemic toxicity (LAST) or resuscitation team involvement via a cardio-respiratory arrest notification, were documented.
Patients were defined as receiving extrapleural LA continuous infusion (CI) if there were initially prescribed CI at time of operation, and received less than 10% of their subsequent 24-hour period LA dose by rescue boluses ordered by our acute pain service. This 10% proportion of bolus doses was established to account for our rescue bolus protocol as defined above.
The primary outcome measure was comparison of the effect of PIB vs. CI on maximum daily 11-point numerical rating scale (NRS-11) ratings as determined by multivariate linear regression analysis, corrected for OMEDD use, total daily LA dose regardless of CI, PIB or rescue bolus route, surgery type, age, opioid PCA type, and use of ketamine analgesia. Secondary outcome measures were the effect of the above covariates on OMEDD use, and the effect of total ‘rescue’ LA boluses on the above outcome measures, by multivariate linear regression. We also performed univariate analyses of the above outcomes and variables to assist the quantification of the possible effect sizes of statistically significant covariates.
2.1. Statistical Methodology
Covariates for the multivariate linear regression model were selected manually based on the likelihood of clinical influence on the outcome of interest. Sample size for the regression model was in keeping with recommended limits of one covariate for every ten samples (13). We reported standardised beta coefficients to compare the relative effects of different covariates on the outcome of interest, as well as unstandardised beta coefficients to provide a point estimate of the magnitude of effect of each covariate on the outcome of interest. For univariate analyses, continuous data was tested for normality using the D’Agnostino-Pearson omnibus test, and groups compared using Student’s t-test (two-tailed, P value < 0.05 considered statistically significant). A power calculation based on a previous audit, (mean OMEDD requirements 167 mg, SD 80mg postoperative day 1) with an alpha of 0.05, beta of 0.8, and an expected decrease of 30%, yielded a sample size of 90 patients. Data analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla California) and SPSS V 21 for Mac (IBM, New York, USA).
3. Results
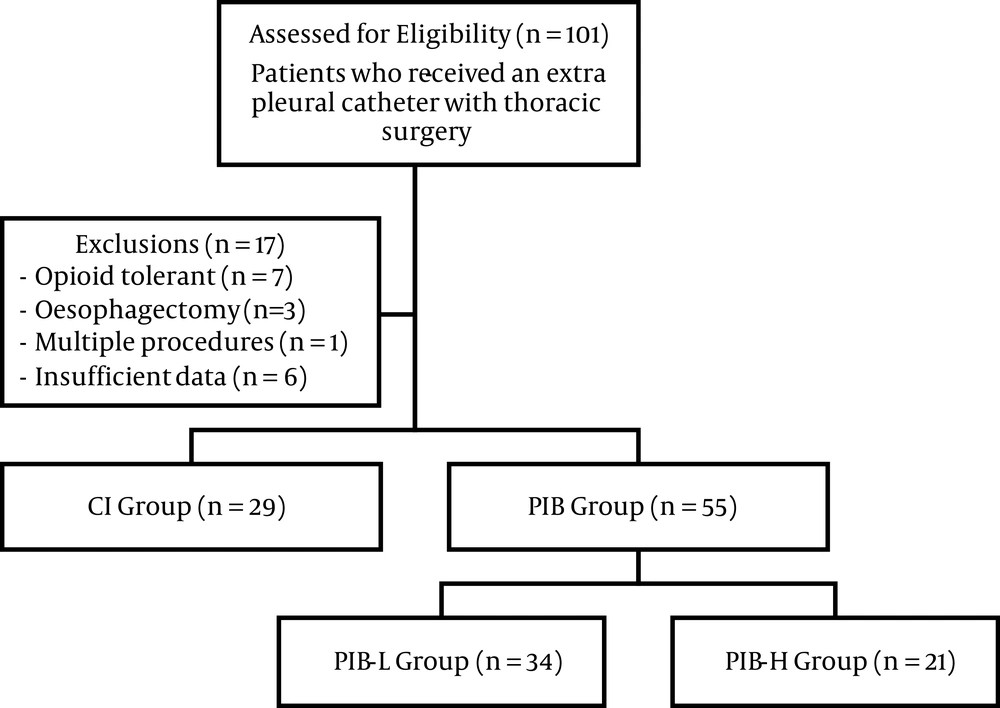
A total of 101 patients received extrapleural analgesia. Overall, 17 patients were excluded, comprising seven patients who were opioid tolerant pre-operatively, three patients who had an oesophagectomy, one patient who underwent multiple procedures, and six patients who had insufficient data. 84 patients were included in the final analysis (Figure 1). No single patient received a higher daily ropivacaine dose than the manufacturer’s recommendation of 770 mg/day (5). Patient demographics are listed in Table 1.
Flow chart of patient assessment, inclusions, and exclusions. POD: post-operative day; n: number; CI: continuous infusion, less than 10% of the total daily ropivacaine dose delivered as a bolus; PIB: programmed intermittent bolus, more than 10% of the total daily ropivacaine dose delivered as a bolus; PIB-L: low dose programmed intermittent bolus, 10% - 25% of the total daily ropivacaine dose delivered as a bolus; PIB-H: high dose programmed intermittent bolus, more than 25% of the total daily ropivacaine dose delivered as a bolus.
| Patient Demographics | Values |
|---|---|
| Age, years | 57.5 ± 15.7 |
| Sex | 40 (47.7) |
| BMI, kg/m2 | 27.0 ± 5.1 |
| Type of surgery | |
| VATS | 41 (49) |
| Thoracotomy | 43 (51) |
| Maximum daily NRS-11 | |
| POD 1 | 6.5 ± 2.2 |
| POD 2 | 5.6 ± 2.0 |
| POD 3 | 5.3 ± 2.5 |
| Minimum daily NRS-11 | |
| POD 1 | 0.7 ± 1.1 |
| POD 2 | 0.6 ± 0.9 |
| POD 3 | 0.3 ± 0.7 |
| Daily OMEDD requirements, mg | |
| POD 1 | 142 ± 116 |
| POD 2 | 112 ± 107 |
| POD 3 | 88 ± 98 |
| Primary opiate on post-operative day 1 | |
| Fentanyl IV | 28 (32) |
| Morphine IV | 24 (28) |
| Oxycodone IV | 14 (17) |
| Oxycodone oral | 28 (33) |
| Total daily dose of ropivacaine, mg | |
| POD 1 | 491 ± 113 |
| POD 2 | 502 ± 18 |
| POD 3 | 528 ± 102 |
Abbreviations: BMI, body mass index; CI, continuous infusion (less than 10% of the total daily ropivacaine dose delivered as a bolus); PIB, programmed intermittent bolus (more than 10% of the total daily ropivacaine dose delivered as a bolus); POD, post-operative day; SD, standard deviation.
aValues are expressed as mean ± SD or No. (%).
Use of PIB on day 0 was associated with reduced maximum daily NRS-11 ratings [standardized/ [unstandardized] beta coefficient -0.34/ [-0.92 NRS-11 if PIB] (P = 0.007). Of our secondary outcome measures, a higher proportion of LA given as PIB on day 1 was also associated with lower maximum daily NRS-11 ratings on day 1 [-0.26/ -0.029 NRS-11 per mg/kg extrapleural bolus ropivacaine] (P = 0.03)]. OMEDD use on day 2, however, was associated with slightly higher maximum NRS-11 ratings [0.28/ +0.006 NRS-11 per mg OMEDD (P = 0.036)]. Age was the only statistically significant factor in the multivariate linear model affecting OMEDD use on day 0 - 2 [day 0: -0.58/ [-4.4 OMEDDs per year of age] (P ≤ 0.005); day 1: -0.49/ [-3.56 OMEDDs per year of age] (P ≤ 0.005); day 2: -0.32/ [-1.9 OMEDDs per year of age] (P = 0.04)].
See Tables 2 and 3 for results of the multivariate linear regression modelling for covariates affecting maximum daily NRS-11 and OMEDD use, respectively.
| Outcome Variable: Daily Maximum NRS | Covariates Examined | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Gender a | Open vs. VATS Surgerya | Ketamine Infusiona | PCA Opioid Type | PIB as Default LA Prescriptiona | Daily OMEDD Use, mg, PO | Total LA Rescue Boluses, mg/kg/day | Total Daily Ropivacaine Dose, mg/kg/day | % Daily Ropivacaine Dose, PIB | |
| Day 0 | ||||||||||
| Unstandardized coefficientsb | 0.023 | -0.692 | 0.548 | -0.641 | -0.17 | -0.924 | 0.011 | 59.919 | -3.929 | -0.022 |
| Standardized coefficientsc | 0.154 | -0.155 | 0.123 | -0.103 | -0.064 | -0.338 | 0.482 | 0.168 | -0.2 | -0.18 |
| P value | 0.175 | 0.132 | 0.232 | 0.371 | 0.545 | 0.007d | 0 | 0.111 | 0.086 | 0.13 |
| Day 1 | ||||||||||
| Unstandardized coefficients | -0.009 | -0.374 | 0.685 | 0.044 | -0.408 | 0.577 | 0.003 | -0.085 | -0.033 | -0.029 |
| Standardized coefficients | -0.064 | -0.086 | 0.158 | 0.007 | -0.155 | 0.219 | 0.156 | -0.006 | -0.03 | -0.257 |
| P value | 0.654 | 0.449 | 0.181 | 0.954 | 0.182 | 0.061 | 0.298 | 0.955 | 0.801 | 0.027d |
| Day 2 | ||||||||||
| Unstandardized coefficients | -0.026 | 0.338 | -0.109 | -0.186 | -0.369 | -0.141 | 0.006 | 0.539 | -0.021 | -0.007 |
| Standardized coefficients | -0.2 | 0.084 | -0.027 | -0.035 | -0.147 | -0.058 | 0.281 | 0.025 | -0.022 | -0.058 |
| P value | 0.129 | 0.488 | 0.83 | 0.786 | 0.251 | 0.659 | 0.036d | 0.838 | 0.864 | 0.653 |
aSignifies binary covariate, if binary factor is present, there is an increase of (β-coefficient x dependent variable unit) in the dependant variable unit value. For negative β-coefficients, the dependant variable similarly decreases.
bUnstandardized coefficients, covariates (a, b, c, etc.) affect the dependent variable (y) by Y = constant + (a) × [coefficienta] + (b) × [coefficientb].
cStandardized coefficients, coefficients are proportionally reduced or increased according to their range of values to allow comparison between covariates.
dP value < 0.05.
| Outcome Variable: Daily OMEDD Use | Covariates Examined | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, y | Gender a | Open vs. VATS Surgerya | Ketamine Infusiona | PCA Opioid Type | PIB as Default LA Prescriptiona | Total LA Rescue Boluses, mg/kg/day | Total Daily Ropivacaine Dose, mg/kg/day | % Daily Ropivacaine Dose, PIB | |
| Day 0 | |||||||||
| Unstandardized coefficientsb | -4.426 | 35.84 | 33.958 | 34.427 | -7.393 | 14.581 | 0.795 | 3.253 | -0.191 |
| Standardized coefficientsc | -0.578 | 0.157 | 0.149 | 0.093 | -0.054 | 0.104 | 0.077 | 0.056 | -0.032 |
| P value | < 0.0005d | 0.103 | 0.131 | 0.338 | 0.584 | 0.281 | 0.426 | 0.581 | 0.738 |
| Day 1 | |||||||||
| Unstandardized coefficients | -3.551 | 24.326 | 35.667 | 47.82 | -4.04 | 9.43 | 37.512 | -0.885 | -1.127 |
| Standardized coefficients | -0.491 | 0.111 | 0.163 | 0.162 | -0.029 | 0.07 | 0.031 | -0.017 | -0.172 |
| P value | < 0.0005d | 0.293 | 0.137 | 0.149 | 0.787 | 0.527 | 0.77 | 0.878 | 0.116 |
| Day 2 | |||||||||
| Unstandardized coefficients | -1.974 | -11.364 | 13.429 | 33.704 | -4.282 | 2.954 | -77.155 | -2.698 | -1.49 |
| Standardized coefficients | -0.319 | -0.057 | 0.067 | 0.123 | -0.033 | 0.025 | -0.071 | -0.058 | -0.274 |
| P value | 0.04d | 0.684 | 0.664 | 0.457 | 0.831 | 0.871 | 0.625 | 0.691 | 0.069 |
aSignifies binary covariate, if binary factor is present, there is an increase of (β-coefficient x dependent variable unit) in the dependant variable unit value. For negative β-coefficients, the dependant variable similarly decreases.
bUnstandardized coefficients, covariates (a, b, c, etc.) affect the dependent variable (y) by Y = constant + (a) × [coefficienta] + (b) × [coefficientb].
cStandardized coefficients, coefficients are proportionally reduced or increased according to their range of values to allow comparison between covariates.
dP value < 0.05.
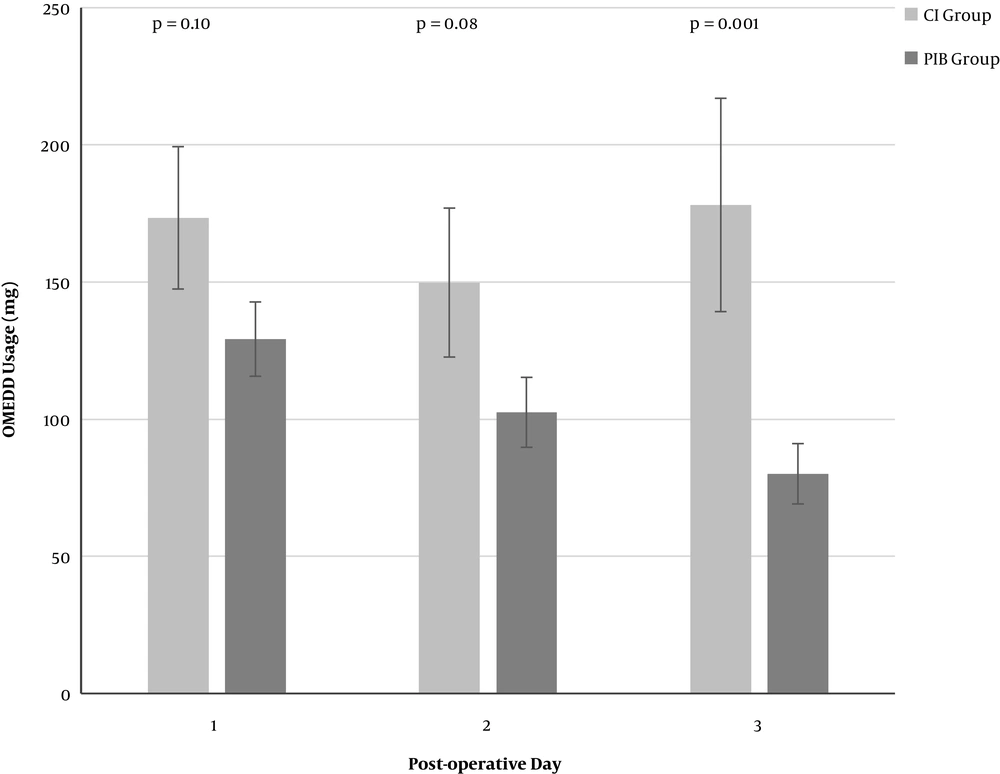
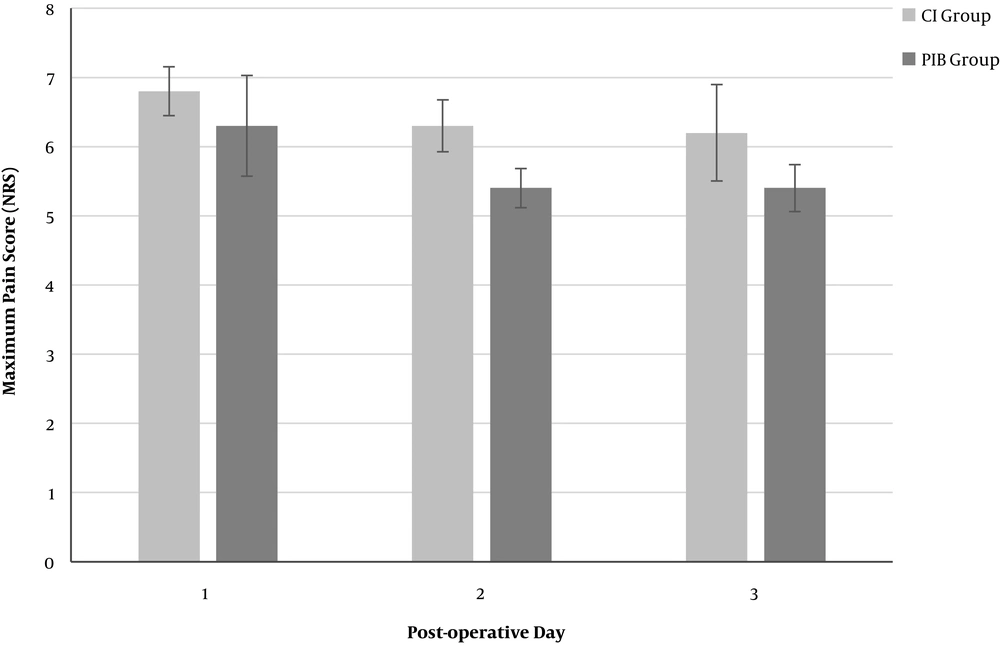
Mean maximum daily NRS-11 pain ratings were highest day 0 (6.4) improving to day 3 (5.7), whilst mean minimum daily NRS-11 ratings over the same period were (1.1) and (0.3). Mean length of stay was 6 days. On univariate analysis, use of PIB appeared to have a significant increasing effect on lower OMEDD use as postoperative days progressed [largest difference day 3 (mean/SD: 98 mg/25 mg); Figure 2]. Similarly, PIB appeared to have a modest effect on reducing NRS-11 [largest difference postoperative day 2: (mean/SD 1.1/0.7); Figure 3].
A comparison of the mean post-operative OMEDD consumption between the continuous infusion (CI) group and the programmed intermittent bolus (PIB) group on each post-operative day (POD). CI: continuous infusion, less than 10% of the total daily ropivacaine dose delivered as a bolus; PIB: programmed intermittent bolus, more than 10% of the total daily ropivacaine dose delivered as a bolus; OMEDD: oral morphine equivalent daily dose; mg: milligrams; POD: post-operative day. Error bars are mean ± standard error of the mean (SEM).
A comparison of mean post-operative numerical rating scale (NRS) pain scores between the continuous infusion (CI) group and the programmed intermittent bolus (PIB) group on each post-operative day (POD). CI: continuous infusion, less than 10% of the total daily ropivacaine dose delivered as a bolus; PIB: programmed intermittent bolus, more than 10% of the total daily ropivacaine dose delivered as a bolus; NRS-11: 11-point Numerical Rating Scale. Error bars are mean ± standard error of the mean (SEM).
3.1. Adverse Events
There were three MET calls and one code blue noted in this patient population throughout the study period. One code blue was called for a patient in the CI group, which was secondary to a vasovagal episode with complete resolution. Two patients in the PIB group had MET calls for type II respiratory failure in the setting of severe underlying pulmonary disease (chronic obstructive pulmonary disease, and granulomatosis with polyangiitis, respectively), both of which resulted in unplanned admissions to the intensive care unit. A third patient in the PIB group required a MET call for supraventricular tachycardia which resolved with intervention on the ward. Outside of MET calls and code blues, there was only one additional adverse event with a patient in the CI group experiencing peri-oral numbness and tingling. The LA infusion was immediately ceased and symptoms subsequently resolved.
4. Discussion
We report results from a retrospective cohort of thoracic surgery patients in a single-centre tertiary teaching hospital who received extrapleural LA by either continuous infusion (CI) or programmed intermittent bolusing (PIB). In keeping with our primary outcome hypothesis, extrapleural LA boluses, whether as part of default prescription of PIB from time of surgery, or total proportion of boluses of LA per daily LA amount, were associated with lower maximum daily NRS-11 ratings. Moreover, the point estimate of the largest effect size of PIB over the study period on maximum daily NRS-11 is remarkably similar between the univariate and multivariate analyses (approximately 1.1 vs. 0.9 NRS-11 units respectively). This is consistent with no other significant patient, surgical or analgesic factors affecting daily maximum NRS-11 ratings in our multivariate model (Table 2), including accounting for total daily LA doses. Conversely, whilst PIB appeared to significantly lower OMEDD use against CI in the univariate analysis, the lack of effect of PIB vs. CI in our multivariate model suggests that other confounding factors had greater influence on the observed difference in OMEDD use.
Two prospective randomised trials have compared paravertebral PIB to CI in thoracic surgery. Fibla et al.'s (10) study showed no difference, whereas Catala et al. (14) found PIB to be inferior. We believe our study’s findings warrant further prospective investigation as the bolus frequency in these prior studies was much lower at 6 hourly, and were without a continuous background infusion. Moreover, in both Fibla et al. and Catala et al.’s work the LA catheters were paravertebral rather than extrapleural. Whilst it is commonly accepted that these spaces are identical, controversy exists over the ability of a percutaneously placed paravertebral catheter to be reliably positioned relative to the endothoracic fascia, potentially affecting LA analgesic efficacy (15, 16). This limitation does not exist for surgically placed extrapleural LA catheters as the endothoracic fascia is on view. The variability in outcome observed when PIB vs. CI via regional analgesia catheters for non-thoracic surgery has been studied and has been proposed by investigators to be due to LA volume rather than absolute dose, a factor which may have influenced our findings in favour of PIB (7-9).
Although the overall maximum daily NRS-11 pain ratings gave the appearance of suboptimal analgesia, these assessments would most likely be during times of deep breathing and coughing; the minimum daily NRS-11 pain assessments in our study showed that at rest, most patients had none, or insignificant pain (Table 1). Whilst not powered to detect differences in adverse outcomes, we were reassured to observe that most critical incidences in the study patients were not related to local anaesthetic systemic toxicity (LAST), and that the one patient with suspected symptoms of high plasma LA concentrations was in fact receiving CI, not PIB. Although only a single incident, this is particularly reassuring as LAST is proposed to be more strongly related to sudden increases in, rather than absolute values of, plasma LA concentrations (17).
Whilst patient age was not a statistically significant covariate affecting NRS-11 pain ratings in our study, there was a profound inverse relationship with OMEDD use. The phenomenon of reduced opioid doses required to achieve given levels of analgesia in older patients is well-recognised (18), and the magnitude of this effect (up to 2-4 times more pharmacodynamic efficacy than in younger patients) (19) may explain the profound effect patient age had in our study compared to the primary intervention of interest.
Our finding of the negative effect of increasing OMEDD use on NRS-11 pain ratings appears counter-intuitive; however emerging evidence supports psychological factors such as anxiety, depression and pain catastrophising as patient factors associated with both higher postoperative OMEDD use and higher reported pain ratings (20) particularly where PCA allows on-demand patient initiated opioid dosing (21). This was not a patient factor accounted for in our study and may explain the observed findings.
This study is limited by its small sample size and single-centre retrospective cohort nature. However, our hospital has all the typical characteristics of many tertiary institutions’ thoracic units, and the surgical and anaesthesia perioperative protocols adopted by our centre are aligned with those in many other tertiary centres. Our findings on multivariate modelling assume a linear relationship between covariates and outcomes, which may not reflect the pattern of response of the variables analysed. Whilst our sample size has adhered to the recommended ratio of covariates analysed to number of samples (13), all samples were required to generate the regression equation, leaving none to submit to model testing. The retrospective nature of the data included could also introduce sources of error in the variables recorded.
We did not include endpoints of length of stay or other major postoperative complications as the retrospective nature of the study and the available sample size limited the capacity to detect changes in these variables. Our finding of an average reduction in NRS-11 pain ratings of approximately 1.0, whilst statistically significant, may not be viewed as clinically significant in this context. For this reason and all afore-mentioned limitations, our findings should be considered hypothesis-forming, and further randomised prospective work would serve well in elucidating the effect of PIB vs. CI on other clinically important and patient-centred outcomes. As the ideal programmed intermittent bolusing regimen for extrapleural analgesia remains to be determined (22), we believe our study adds direction to further prospective work on the efficacy of higher-frequency PIB for thoracic surgical postoperative analgesia.
4.1. Conclusions
Use of an extrapleural local anaesthetic programmed intermittent bolus regime with initial prescription of ropivacaine 0.2% at 5 mL/h background infusion and boluses of 6 mL every 2 hours for acute pain after thoracic surgery is associated with a modest reduction in maximum daily NRS-11 pain assessments, when compared against initial prescription extrapleural continuous infusion of ropivacaine 0.2% at 10 mL/h, after adjusting for total local anaesthetic dose, and adjunctive analgesic, surgical and patient factors. Consistent with prior published data, patient age was the strongest analysed factor affecting pain outcomes, with a profound effect on postoperative opioid requirements; conversely, increased opioid usage via patient-controlled analgesia was associated with higher NRS-11 pain assessments. Further randomised prospective work is required to confirm the beneficial association of PIB and improved postoperative pain outcomes.