1. Background
Chronic pain is considered the world’s third-largest health problem (1) and has a great impact on many health domains including physical, emotional, and social health. It also extensively affects the quality of life (QOL) (2-6). Inadequate pain management (IPM) has been widely reported (7-10). A recent review suggested that nearly 50% of chronic pain patients did not receive adequate pain management (11-14). Several factors have been proposed to contribute to the IPM including patient-related factors (age, sex, education, social and psychological status) (15-18), healthcare provider-related factors (underestimation of pain intensity, lack of adequate training) (19-21), and disease-related factors (pain intensity, benign vs. malignant disease) (2, 3). Meanwhile, other studies proposed that inadequate pain assessment is the cornerstone of under-treatment (22, 23).
Pain management adequacy can be assessed by the patient’s satisfaction, pain intensity, the extent of pain relief, and pain interference with life. Pain management index (PMI) is a well-validated mean for assessing the adequacy of pain treatment (7) in a large variety of chronic pain conditions (24, 25). The PMI compares the patient’s reported level of pain intensity with the potency of prescribed analgesics.
A few studies have attempted to find predictors (risk factors) of IPM. Many variables such as personal characteristics, inter-individual relationships, employment status, and pain features may have significant correlations with IPM. Older patients, women, ethnic minorities, and less educated patients are at risk of IPM (15-18). However, the majority of the studies have been conducted with small sample size. Many reports lack the simultaneous evaluation of different variables in a model. In addition, there are inconsistencies in the results of these studies about the role of different factors like age and sex.
2. Objectives
We aimed to evaluate the prevalence of IPM in a sample larger than the samples of the previous studies. Furthermore, we considered more possible confounding factors in IPM by simultaneously applying less-studied variables (such as education level, employment status, marital and household conditions). As there is a relationship between obesity and higher pain intensity (26-28), we considered a new variable, i.e. the body mass index (BMI), in logistic models to demonstrate more different factors that may independently correlate with IPM. Moreover, most of the studies focused on IPM in cancer patients (7-9, 11) whereas, we evaluated pain management in a wide variety of chronic pain conditions.
3. Methods
The Ethics Committee of the University approved this cross-sectional study. All eligible patients signed informed consent forms. The inclusion criteria were as follows: (1) all consecutive patients who referred to our pain clinic between 2014 and 2017; (2) patients aged 15 to 90 years; (3) patients with chronic pain (pain duration ≥ 3 months), and (4) patients with documented records indicating the type and dose of analgesics they had already received in primary and/or secondary care centers. Patients younger than 15 years, with a documented psychiatric disorder, or not willing to answer the questions were excluded. Patients who completely met the eligibility criteria were enrolled in the study. By considering the IPM prevalence lower limit of 30% and the maximum acceptable error level of 0.05, we would need at least 504 patients to detect this prevalence with a precision of 4%.
The patients filled in questionnaires. The questions were about age, sex, weight, height, employment status, education level, marriage (or being in a serious relationship), household condition, and the number of children. The patients answered the questions about pain intensity, pain duration, and the type and dosage of previous analgesics they were taking at the first visit. If any patient had difficulty to answer the questions, a trained staff was always available to give help. The staff ensured that the patients had completely understood and answered the questions. Afterward, the attending pain specialist visited the patients. During the consultation, the specialist evaluated the patients’ previous pain treatments and records including the type and dosage of previous medications. He also verified the questionnaire to be adequately completed. If there were any defects in a questionnaire, he would address the unanswered questions. The interviews were to support the validity of the data provided by self-report. We used the baseline data to assess the adequacy of pain management in the primary and secondary care centers.
3.1. Measures
Age was classified into four groups of ≤ 30, 31 - 45, 46 - 65, and > 65 years. Education was categorized into levels including illiterate, less than high school, high school graduation, bachelor (college graduation) or lower, and above bachelor. The BMI was divided into four groups of underweight (BMI < 18.5), normal (BMI = 18.5 - 24.9), overweight (BMI = 25 - 29.9), and obese (BMI > 30).
We employed the validated Persian version of the brief pain inventory (BPI) for pain assessment (29). The pain intensity (PI) was measured by an 11-point numeric rating scale (NRS) from having no pain at all (score 0) to having the worst imaginable pain (score 10). The patients reported the PI at its lowest and highest levels and at the time of the interview (present PI). They also reported overall (average) pain intensity during the last week. We calculated the mean PI as the mean value of the present, highest, and overall PI and recorded it as the mean PI for each patient. The mean PI was also categorized into four scores, including score 0 = no pain (NRS: 0), score 1 = mild pain (NRS: 1 - 3), score 2 = moderate pain (NRS: 4 - 7), and score 3 = severe pain (NRS: 8 - 10) (11). There are various versions of the PMI calculation (11). We employed the Zelman version of PMI that was calculated based on the potency of used analgesics and the mean PI for each patient (9). The WHO guidelines categorize (30) the analgesics into four classes based on their potency, including medications with no analgesic effect (analgesic score: 0), non-opioid analgesics such as NSAIDs (analgesic score: 1), weak opioids (analgesic score: 2), and strong opioids (analgesic score: 3). For patients taking multiple analgesics, the score was related to the highest analgesic class that the patients were taking at the time of the interviews (1). A trained physician reviewed and classified the analgesics. Again, another trained pain specialist checked the classification provided by the first physician. The Zelman PMI, then, was calculated by subtracting the mean pain intensity score for each patient from the analgesic score (7). Therefore, the PMI could range from -3 (patients with severe pain receiving no analgesics) to +3 (patients without pain receiving a strong opioid). Negative PMI showed incongruence between the PI and analgesic potency scores (pain stronger than medication), indicating a case of IPM. Meanwhile, 0 or positive scores expressed acceptable pain management (medication potency above pain intensity). The PMI is not a perfect indicator of the adequacy of pain management. Its limitations will be explained in the limitation section.
3.2. Statistical Analysis
Continuous variables were shown as the mean (± standard deviation [SD]). The categorical variables were presented as frequency and percentage. The chi-squared test assessed the distribution of adequate pain management and IPM across the subgroups of patients with different levels of PI. No sampling-related analysis consideration was applied.
We used a logistic regression model based on a step-wise approach to detect parameters that independently and significantly were associated with the adequacy of pain management. In the first step, personal, family, and socioeconomic parameters were assessed. They included age, sex, employment status, education, marital status, household condition, and the number of children. Parameters with potential associations were detected by univariable logistic analysis and they were entered into a multivariable model to adjust for probable confounding and suppressing effects. Parameters that retained their significant associations in the multivariable model and/or added to the fitness of the model were selected. In the second step, the developed multivariable model was further adjusted for clinical parameters with potential associations. They were selected from BMI and pain duration. Plausible interaction terms among final determinants were also checked. We also considered a sensitivity analysis and used logistic regression analysis to exclusively investigate the correlation of PI with analgesic potency, separately. In the case of analgesic potency, the factors determining the odds of receiving opioids (weak or strong) versus other medications (non-opioids or non-analgesics) were analyzed. For PI, factors associated with the odds of having severe PI versus lower PI were analyzed. We used Stata/SE V. 11.1 (Stata Corp LP, USA) for statistical analysis. Except for screening analyses, a P value of < 0.05 was considered significant.
4. Results
Consecutive cases (576 patients) during 2014 - 2017 were interviewed for eligibility criteria and 511 were recruited. Among 65 patients who were excluded, 45 had a documented psychiatric disorder and the remaining 20 patients could not provide clear, documented, and detailed information about the analgesic therapy they had received. All of the 511 eligible patients filled in questionnaires and their data were analyzed (Figure 1). The average age of the patients was 47.9 years (SD = 14.9) and 214 patients (42%) were male. Table 1 presents the descriptive data of the patients. Cases included in the final analysis had no missing data in the main study parameters.
| Characteristics | Values |
|---|---|
| Age, y | |
| ≤ 30 | 65 (12.72) |
| 31 - 45 | 168 (32.88) |
| 46 - 65 | 200 (39.14) |
| > 65 | 78 (15.26) |
| Gender | |
| Male | 214 (41.88) |
| Female | 297 (58.12) |
| Employment status | |
| Employed | 199 (38.94) |
| Unemployed | 312 (61.05) |
| Education | |
| Illiterate | 54 (10.57) |
| Less than high school | 195 (38.16) |
| High school graduate | 142 (27.79) |
| Bachelor or lower | 98 (19.18) |
| Above bachelor | 22 (4.30) |
| Marriage | |
| Single | 102 (19.96) |
| Married | 409 (80.04) |
| Household condition | |
| Alone | 31 (6.07) |
| Living with someone else | 480 (93.93) |
| Number of children | |
| 0 | 46 (9.00) |
| 1 - 2 | 194 (37.96) |
| > 2 | 271 (53.03) |
| Pain duration, months | 60.52 ± 89.00 |
| Pain intensityb | |
| Minimum | 2.91 ± 2.36 |
| Maximum | 8.50 ± 1.68 |
| At the time of the interview (present) | 5.35 ± 2.36 |
| Overall (average) | 6.87 ± 1.37 |
| Mean PIc | |
| Mild | 39 (7.63) |
| Moderate | 340 (66.54) |
| Severe | 132 (25.83) |
| BMI, kg/m2 | |
| Underweight | 29 (5.70) |
| Normal | 115 (22.59) |
| Overweight | 309 (60.70) |
| Obese | 56 (11.00) |
| Analgesic Potencyd | |
| Non-analgesics | 62 (12.13) |
| Non-opioid analgesics | 334 (65.36) |
| Weak opioids | 65 (12.72) |
| Strong opioids | 50 (9.78) |
Abbreviation: BMI, body mass index.
aValues are expressed as mean SD or No. (%).
bPain intensity was evaluated by NRS.
cMean pain intensity for each patient equal to the mean values of the present, highest, and average pain intensities for each patient.
dNumber of patients receiving different types of analgesics.
The mean values of minimum, present, maximum, and overall (average) pain intensities of the patients were 2.91, 5.35, 8.5, and 6.87, respectively. Moreover, 472 (92.3%) patients had moderate to severe pain intensity and 39 (7.6%) patients experienced mild pain. The mean duration of chronic pain was more than five years, i.e., 60.52 months (SD = 89), prior to the consultation at the pain clinic. In addition, 449 patients (88%) were prescribed with some kinds of analgesics (Table 1). An acceptable PMI, which included PMI scores of 0 and 1, was reported only in 120 (23%) patients. A negative PMI (scores of -1 to -3) was calculated in 77% of the patients. Only 10% of the patients with severe pain received a strong opioid. Table 2 shows the correlation between the mean PI and the analgesic potency with the Zelman PMI. The percentage of patients with negative PMI was significantly higher in patients with severe mean PI than in patients with mild pain (P < 0.001).
| Zelman PMI | Number of Patients with Different Mean PI | Number of Patients Using Different Potencies of Analgesicsc | |||||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | 0 | 1 | 2 | 3 | |
| Acceptable | 32 (82.05) | 77 (22.65) | 11 (8.33) | 0 (0.00) | 27 (8.08) | 43 (66.15) | 50 (100) |
| Inadequate | 7 (17.95) | 263 (77.35) | 121 (91.67) | 62 (100.00) | 307 (91.92) | 22 (33.85) | 0 (0.00) |
| Total | 39 (100) | 340 (100) | 132 (100) | 62 (100) | 334 (100) | 65 (100) | 50 (100) |
Abbreviations: Mean PI, the mean of the present, highest, and average pain intensities for each patient, categorized into mild, moderate, and severe; PMI, pain management index.
aValues are expressed as No. (%).
bPatients were classified based on different groups of mean PI and analgesic potency they were using. Chi-square P value < 0.001 for the correlation of PMI with mean PI or analgesic potency.
cAnalgesic potency: 0 (non-analgesic), 1 (non-opioid), 2 (weak opioid), and 3 (strong opioid).
Univariable logistic analysis revealed that age (P = 0.02), sex (P = 0.007), education level (P = 0.01), and BMI (P = 0.03) were significantly correlated with IPM. No association was detected between negative PMI and employment status (P = 0.24), marital status (P = 0.71), household conditions (P = 0.89), the number of children (P = 0.57), and pain duration (P = 0.49).
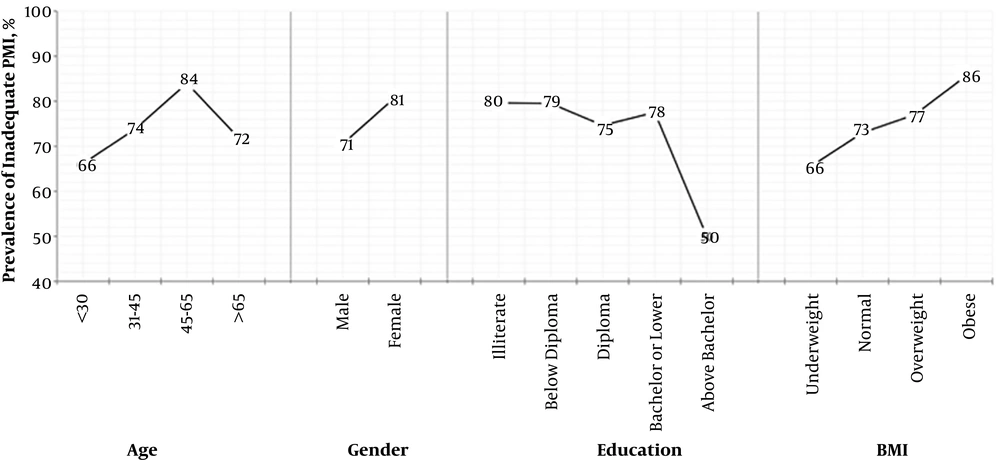
Next, we developed multivariable logistic regression models to investigate parameters with independent and significant associations with negative PMI. In the first multivariable logistic model, age, sex, and education level retained significant associations with IPM (Table 3); this model was further adjusted for BMI, a clinical parameter that showed a potential association with negative PMI in univariable analysis. The second model (Table 3) demonstrated that women (odds ratio (OR) = 1.62, 95% confidence interval (CI): 1.03 - 2.54) and patients aged 45 - 65 years (OR = 2.34, 95%CI: 1.16 - 4.73) had higher odds to receive IPM than their corresponding reference groups. It also indicated that patients with higher education (a degree higher than bachelor) had significantly lower odds to have IPM than illiterate patients (OR = 0.32, 95%CI: 0.1 - 0.89). Obesity was associated with up to three-fold higher risk of IPM (OR = 3.04, 95%CI: 1.03 - 8.51). Figure 2 demonstrates the prevalence of negative PMI in different groups of patients.
| Determinant Parameter, Subgroup(s) | Univariable Logistic Analysis | Multivariable Logistic Models | |||
|---|---|---|---|---|---|
| Adequate PMI (N = 120) | Inadequate PMI (N = 391) | OR (95% CI) | ORc (95% CI) | ORd (95% CI) | |
| Age | |||||
| ≤ 30 | 22 | 43 | Reference | Reference | Reference |
| 31 - 45 | 44 | 124 | 1.44 (0.73 - 2.78) | 1.66 (0.85 - 3.23) | 1.42 (0.71 - 2.85) |
| 45 - 56 | 32 | 168 | 2.69 (1.42 - 5.08)* | 2.71 (1.36 - 5.37)* | 2.34 (1.16 - 4.73)* |
| > 65 | 22 | 56 | 1.30 (0.60 - 2.82) | 1.31 (0.55 - 3.10) | 1.11 (0.46 - 2.71) |
| Gender | |||||
| Male | 63 | 151 | Reference | Reference | Reference |
| Female | 57 | 240 | 1.76 (1.16 - 2.65)* | 1.64 (1.06 - 2.56)* | 1.62 (1.03 - 2.54)* |
| Education | |||||
| Illiterate | 11 | 43 | Reference | Reference | Reference |
| Below diploma | 40 | 155 | 0.99 (0.42 - 2.18) | 1.07 (0.47 - 2.44) | 1.04 (0.45 - 2.39) |
| Diploma | 36 | 106 | 0.75 (0.32 - 1.69) | 0.71 (0.30 - 1.69) | 0.65 (0.27 - 1.57) |
| Bachelor or lower | 22 | 76 | 0.88 (0.35 - 2.12) | 1.11 (0.44 - 2.85) | 1.10 (0.41 - 2.79) |
| Above bachelor | 11 | 11 | 0.26 (0.08 - 0.84)* | 0.32 (0.01 - 0.94)* | 0.32 (0.1 - 0.89)* |
| BMI | |||||
| Underweight | 10 | 19 | Reference | Reference | |
| Normal | 31 | 84 | 1.43 (0.60 - 3.40) | 1.61 (0.65 - 3.96) | |
| Overweight | 71 | 238 | 1.76 (0.78 - 3.97) | 1.62 (0.69 - 3.78) | |
| Obese | 8 | 48 | 3.16 (1.08 - 9.21)* | 3.04 (1.03 - 8.51)* | |
Abbreviations: BMI, body mass index; CI, confidence interval; N, number; OR, odds ratio; PMI, pain management index.
aVariables not listed in the table did not attain significant associations.
b*: Indicates the ORs with P value < 0.05.
cThis model was adjusted for personal and socioeconomic parameters.
dThis model was further adjusted for clinical parameters (i.e. BMI and pain duration).
We further studied the associations suggested by the multivariable model developed for PMI. The correlations of the mean PI and analgesic potency were separately investigated (Table 4). These associations were also adjusted for the association between the mean PI and analgesic potency. In the model developed for the analgesic potency, obese patients were almost three times less likely to receive analgesics with adequate potency (OR = 0.27, 95% CI: 0.09 - 0.84). In addition, patients aged 45 - 65 years had the highest likelihood to receive analgesics with inadequate potency compared to patients aged 30 or younger (OR = 0.40, 95% CI: 0.19 - 0.81). The sex and education level did not show any significant associations with the potency of analgesics. On the other hand, women were more likely to have severe mean PI (OR = 1.67, 95% CI: 1.08 - 2.59). Furthermore, cases with higher levels of education were less likely to report severe mean PI (OR = 0.33, 95% CI: 0.10 - 0.86). The age and BMI did not show any significant correlation with the mean PI (Figure 3).
| Determinant Parameter, Subgroup(s) | Dependent Variable | |
|---|---|---|
| Analgesic Potency, OR (95% CI) | Mean PI, OR (95% CI) | |
| Age | ||
| ≤ 30 | Reference | Reference |
| 31 - 45 | 0.75 (0.38 - 1.50) | 1.35 (0.64 - 2.88) |
| 45 - 65 | 0.40 (0.19 - 0.81)* | 1.16 (0.55 - 2.45) |
| > 65 | 0.72 (0.29 - 1.80) | 0.98 (0.39 - 2.44) |
| Gender | ||
| Male | Reference | Reference |
| Female | 0.68 (0.42 - 1.17) | 1.67 (1.08 - 2.59)* |
| Education level | ||
| Illiterate | Reference | Reference |
| Less than high school diploma | 1.06 (0.42 - 2.68) | 0.71 (0.36 - 1.40) |
| High school diploma | 2.01 (0.78 - 5.19) | 0.79 (0.38 - 1.64) |
| Bachelor or lower | 1.03 (0.36 - 2.91) | 0.42 (0.18 - 0.97)* |
| Above bachelor | 2.46 (0.67 - 9.04) | 0.33 (0.10 - 0.86)* |
| BMI | ||
| Underweight | Reference | Reference |
| Normal | 0.52 (0.21 - 1.31) | 0.81 (0.30 - 2.18) |
| Overweight | 0.49 (0.21 - 1.17) | 0.84 (0.34 - 2.12) |
| Obese | 0.27 (0.09 - 0.84)* | 0.80 (0.27 - 2.36) |
Abbreviations: BMI, body mass index; Mean PI, the mean value of the present, highest, and average pain intensities for each patient categorized into mild, moderate, and severe.
b*: Indicates the ORs with P value < 0.05.
5. Discussion
About 92% of the patients in this study had moderate to severe pain while 77% of them had IPM. The IPM was significantly more in patients with severe pain. Age, sex, education, and BMI could independently correlate with IPM.
There are numerous reports about the prevalence of IPM in cancer patients (25% - 82%) (7-9, 11, 31-36) whereas a few studies have evaluated the adequacy of pain treatment in non-malignant conditions (24, 25). The high rate of negative PMI (77%) in our study is similar to other reports and shows that IPM occurs in patients experiencing malignant and non-malignant conditions.
There is a controversy about the correlation of independent factors such as age, sex, education, job, and marital status with IPM (13, 32, 34, 36). Greco et al. reviewed 46 papers about IPM (37). Only 6 reports had a sample size with more than 500 patients. The current study is the first report of independent associations of age, sex, education, and BMI with IPM in a large sample of patients (511 cases).
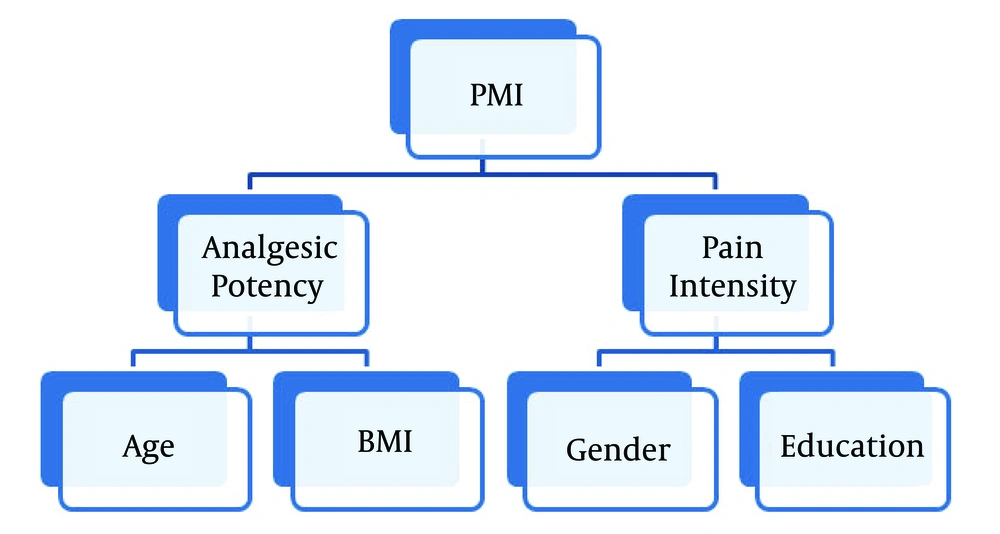
Our study demonstrated that age, sex, education, and BMI could predict the odds of IPM. We investigated the correlation between the mean PI and analgesic potency in the final model (Table 4). Sex and education showed significant associations with the mean PI whereas age and BMI had correlations with analgesic potency.
The relationship of sex with the adequacy of treatment is not consistent in different reports. Some studies showed no correlation between sex and IPM (32, 36, 38), while other reports demonstrated more negative scores of PMI in women (1, 7, 8, 33, 39). Our results revealed that women were 1.6 times more likely to have IPM, which was compatible with some studies (7). Some mechanisms have been proposed to explain this difference, including different pain sensitivities or different responses to analgesics between the two sexes (40-42), as well as sex bias in the physician prescription of potent opioids (33). Our final model demonstrated that the difference was due to different pain intensities reported by women.
Education is a less-studied variable for IPM. Our study revealed that a high level of education was inversely related to the negative PMI (Figure 2), which was compatible with other reports (8, 36). It can be partly due to the different attitudes of illiterate and educated patients toward opioid use and addiction (37). Our final models revealed that the correlation between education and negative PMI was mediated by the reported intensities. Furthermore, illiterate patients who are more populated in rural and less developed areas may have less access to quality pain clinics.
Age is another controversial determinant of negative PMI. Some studies did not find any correlation between age and PMI (13, 33) while the others reported the older age as a protective factor against IPM (32, 34, 36). Some studies demonstrated an association between the younger age (< 40 in some studies and < 65 in others) and better pain management (7, 8, 43). In our study, a higher percentage of negative PMI was observed in the age group of 45 - 65 years (Figure 2). Our final model demonstrated that the age was correlated with PMI via the analgesic potency rather than pain intensity (Table 4). It can be explained that opiophobia and fear of side effects of potent opioids can be a pivotal factor in IPM in older patients. Furthermore, we observed a drop in the prevalence of negative PMI in people older than 65 years old (Figure 2). The pharmacodynamics and pharmacokinetics of medications may change in favor of the reduction of the required dosage of analgesics, especially opioids in older patients. It is proposed to reduce the dose of opioids to 50% in geriatric patients (44). Therefore, older patients may need less analgesic for certain pain intensity. Consequently, a dosage that is insufficient in a younger patient can be considered overtreatment in older patients with the same weight. Hence, in old patients, the opioid requirement decreases and thus IPM can hide behind this change in opioid requirement.
The BMI as a determinant of negative PMI was assessed for the first time in this study. Obese patients were at higher risk of IPM (Figure 2). In addition, our models showed that BMI probably mediated its effect via the potency of analgesics. Previous studies demonstrated that obesity was associated with higher pain levels in patients even after adjustment for other demographic and pain-related factors (26-28). The physicians’ concern for the diverse side effects of potent opioids and using different types of non-opioid analgesics had significant impacts on prescribing analgesics for obese patients. These patients usually have more health issues including fatty liver, hypertension, insulin resistance, diabetes, depression, obstructive sleep apnea, and respiratory compromise (28, 45-47), which may limit the physicians’ decision to prescribe potent opioids for obese patients. Moreover, the volume of distribution and the rate of metabolism/elimination of analgesics are higher in obese patients due to the fatty liver-altered enzymatic activity, which can decrease the efficacy of prescribed drugs (48, 49).
There are some limitations to this study. The PMI is not a perfect indicator of IPM because it does not take into account factors including patients’ compliance, the dosage of medications, route of administration, the potential effect of adjuvant analgesics (antidepressants), and other non-pharmacological modalities. Residual pain intensity despite treatment is probably not an appropriate measure of IPM because the target of chronic pain management is not always to reduce the PI. The improvement of quality of life is also a very important factor. There is a big difference between cancer and non-cancer pain in terms of natural course and treatment strategy. Pain phenotype (neuropathic versus non-neuropathic) is a crucial factor to determine drug efficacy while we evaluated pain management in a wide variety of chronic pain conditions. Our pain clinic is a tertiary and public center; consequently, our patients are not the representatives of the general population. Our patients had non-negligible pain in spite of their prior management. Thus, we can assume that the prevalence of severe pain and IPM would be higher in our patients than in the general population.
5.1. Conclusions
We conclude that the prevalence of IPM was quite high in chronic pain patients, especially in patients with severe pain. Age (45 - 65 y), sex (female), education (above bachelor), and BMI (obese patients) showed significant correlations with IPM. Age and BMI mediated their relationships with negative PMI via analgesic potency; whereas, sex and education mediated their effects by pain intensity.