1. Background
Neuropathic pain arises from pathological abnormalities of the peripheral or central nervous system (1, 2). Neuropathic pain is known to be induced by not only diabetes and cancer but also physical injuries of the nervous system, such as surgery-associated injury. Several animal models have been developed in the past to study the pathophysiological mechanisms underlying neuropathic pain (1-4). The chronic constriction injury (CCI) model of the sciatic nerve is one of them in mice (1, 2). Several behavioral tests have also been devised to assess pain in rodent models, including the sensitivity of the paw evaluated through reflex reactions to heat or mechanical stimuli such as von Frey filaments. In mice, however, because of their high restless activity and responsiveness to humans, it is very hard to give the moving animals consistent stimuli and get consistent, reliable reactions (3, 4).
Sedation is a continuum that levels up from minimal to deep in a dose-response manner (5). This continuum can be classified into levels based on some characteristics: minimal sedation is the condition that subjects are awake but calm; moderate sedation is the condition that subjects are asleep but easily aroused; deep sedation is the condition that subjects are asleep and unarousable (6).
2. Objectives
In deep sedation, subjects show a purposeful response after repeated or painful stimulation. We, thus, hypothesized that deep sedation induced by inhalational anesthesia could reduce animals’ high restless activity and responsiveness to human contact, thereby making us stimulate them consistently to get reliable reactions.
3. Methods
3.1. Animals
After receiving approval from the IRB (Aichi Medical University reference number 2019 - 2020), all experiments were administered following the Guidelines on Ethical Standards for Investigation in Animals (6) and approved by our institution’s Ethics Committee. Experiments were performed on male C57BL/6J mice (aged eight weeks) and prairie voles (aged eight weeks). We used male mice and prairie voles, bred in our animal facility. The animals were set in groups of three to six in cages under a standard 12-h/12-h light/dark cycle; food and water were ad libitum.
3.2. Proper Deep Sedation Test
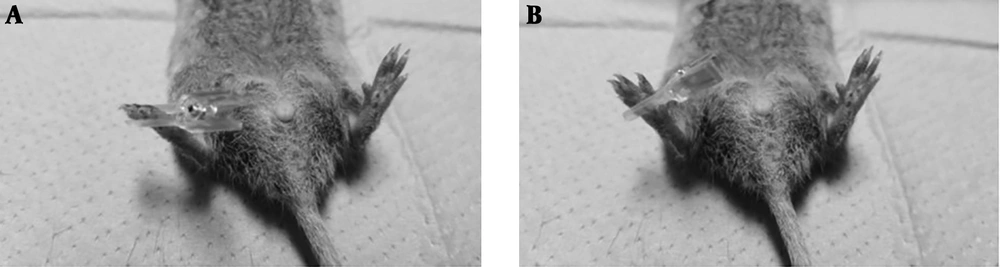
The minimum alveolar concentration (MAC) is the concentration of inhalational anesthetics in the lungs needed to prevent movement in response to a noxious stimulus in 50% of subjects (7). To identify appropriate deep sedation induced by isoflurane anesthesia that prevents animals’ high restless activity and responsiveness to human contact, thereby allowing us to stimulate them consistently to get reliable reactions, we first planned to investigate isoflurane MAC for each animal (five mice and five prairie voles). In a clear plastic chamber, 4.0% isoflurane was induced in 100% oxygen. When losing the righting reflex, the upper part of the animal body was put in a continuously flushed chamber of our own making (2 L/min) where isoflurane in 100% oxygen was administered to the spontaneously breathing animal. The isoflurane concentration was monitored at the chamber gas outlet using an agent analyzer (Datex-Ohmeda, Helsinki, Finland). After an initial equilibration period of 10 min with 2% isoflurane, the MAC analysis started. The concentration of isoflurane was changed by steps of 0.1% and then maintained for 10 min. Then, a five-second noxious stimulus was applied three times at 30-second intervals to the base of the right toes with a noxious arterial clip (the clip exerted a pressure of 350 g/mm2 and was painful when applied to the skin of the experimenter) (Figure 1A and B) (8, 9). A gross movement of the head, trunk, or extremities during the triple stimuli was judged as a positive response. The MAC value of an individual animal was defined by averaging the largest concentration permitting movement and the smallest concentration suppressing movement. The mean (SD) MAC values of mice and prairie voles were 1.5 (0.1) and 1.5 (0.1), respectively. Furthermore, the concentration of isoflurane was reduced at 0.1% increments, and this procedure was continued to 1.2% isoflurane. We observed that it was very hard to stimulate the animals consistently and get consistent and reliable reactions with less than 1.3% isoflurane because of their high restless activity to the noxious stimulus; thus, we defined 1.3% isoflurane anesthesia as appropriate deep sedation.
3.3. Animals and Surgery
Both sham and CCI animals were operated on. The animals were anesthetized using isoflurane inhalation 3%. The right common sciatic nerve was identified at the mid-thigh level through the biceps femoris. In sham animals (five mice and six prairie voles), no ligatures were tied, which meant that sham animals as controls had only sciatic exposure. In contrast, three ligatures (either silk 6.0 or catgut 6.0) were loosely bound around the sciatic nerve of CCI animals (six mice and seven prairie voles) at 1-mm intervals until they provoked a brief twitch in the associated hind paw in CCI animals (1, 2). The muscle layer was then stitched, and the incised skin layer was closed using clips. On day 4, the clips were removed.
3.4. Scoring of Nociceptive Behavior and Mechanical Threshold Test
The pain was inferred from impairment of gait and stance by using the scoring of nociceptive behavior (10) and mechanical threshold test in all animals. Sham animals (five mice and six prairie voles) and CCI animals (six mice and seven prairie voles) were tested before, four days after, and seven days after surgery. First, for the scoring of nociceptive behavior, the animals were set in individual chambers (100 mm × 100 mm, height 200 mm) for 5 min. Each animal was rated using a rating scale previously described: 0, no visible impairment of gait or stance and foot firmly placed flat on the ground with the normal spread of toes; 1, moderate impairment of stance and foot placed on the ground but with toes tightly folded together; 2, severe impairment of gait and stance and foot either entirely elevated from the ground or only the lateral part of the foot very lightly touching the ground and toes tightly pulled together (10). Since the stance frequently changed during the observation period, the highest score maintained for at least 10 s was, therefore, assigned as the final score. Intermediate scores (0.5 and 1.5) were used for occasional animals that displayed a behavior between the above-described definitions. Two independent observers performed the pain scoring in a blinded manner. The scoring results of the two observers were identical.
Next, the threshold of response to the mechanical stimulus was evaluated using arterial clips with incremental pressure (30, 60, 120, 225, and 350 g/mm2 measured by a pressure sensor system). In a clear plastic chamber, 4.0% isoflurane was induced by using 100% oxygen. After losing the righting reflex, the upper part of the animal body was put in a continuously flushed chamber of our own making where 1.3% isoflurane in 100% oxygen (2 L/min) was administered to the spontaneously breathing animal. The isoflurane concentration was monitored at the chamber gas outlet using an agent analyzer (Datex-Ohmeda, Helsinki, Finland). After an initial equilibration period of 10 min with 1.3% isoflurane, each clip was applied to the base of bilateral toes for five seconds three times, beginning with the 30 g clip in a stepwise ascending order after negative responses until a positive response was observed. The threshold was defined as the pressure when a positive response was observed.
3.5. Statistics
Data are presented as the mean (SD). All analyses were carried out with GraphPad prism 5. To analyze the differences between the limbs of CCI and sham animals, the ANOVA test was administered, followed by the Tukey test for multiple comparisons. For the intragroup analysis, the repeated-measures ANOVA test was performed, followed by the Tukey test for multiple comparisons. A P value of < 0.05 was regarded as significant.
4. Results
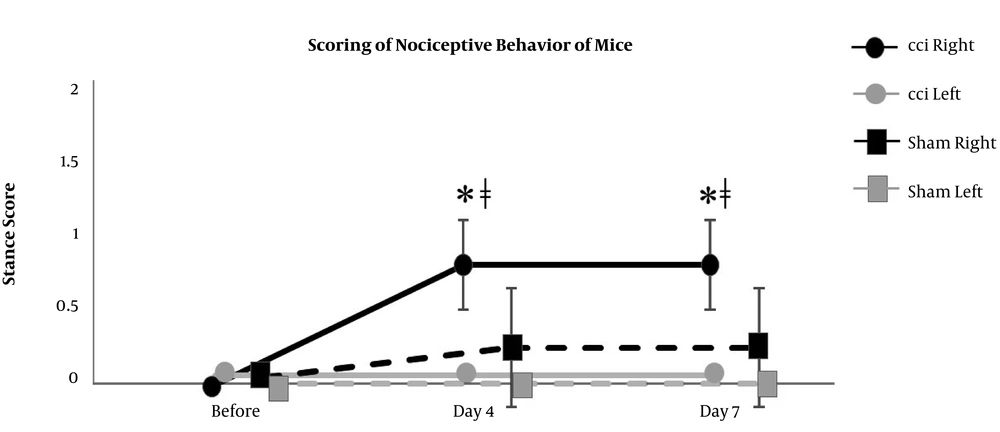
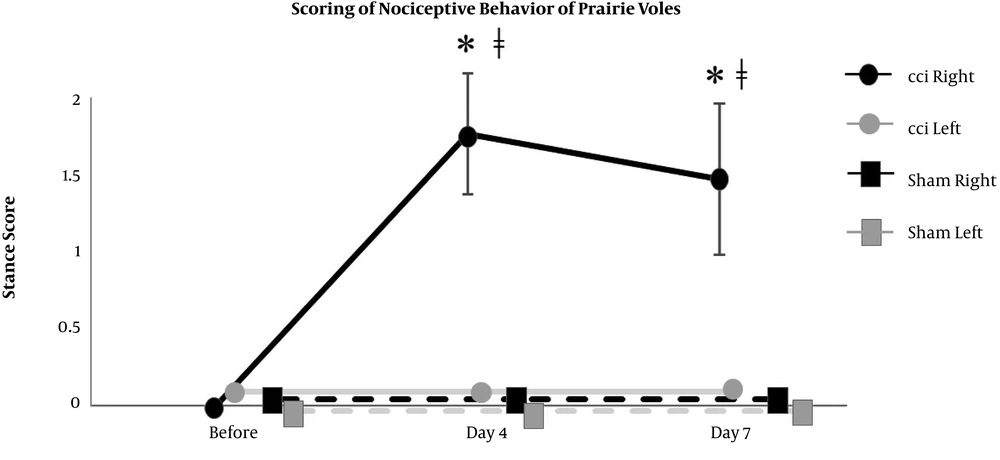
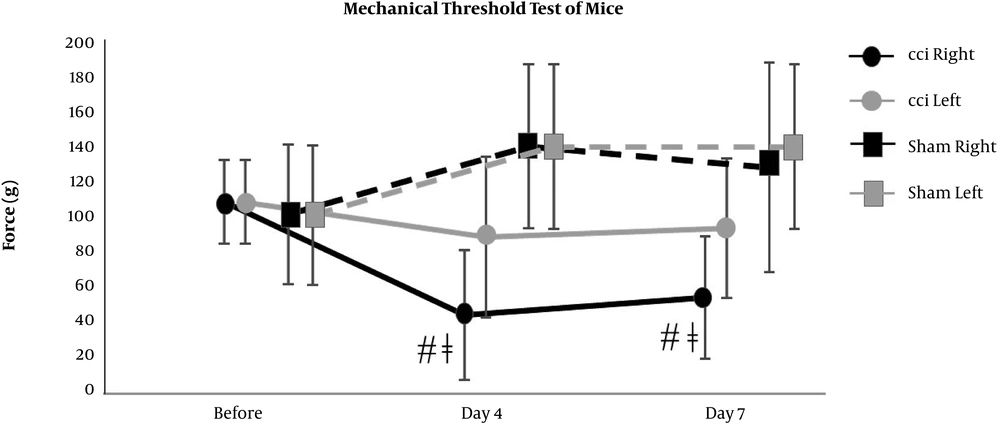
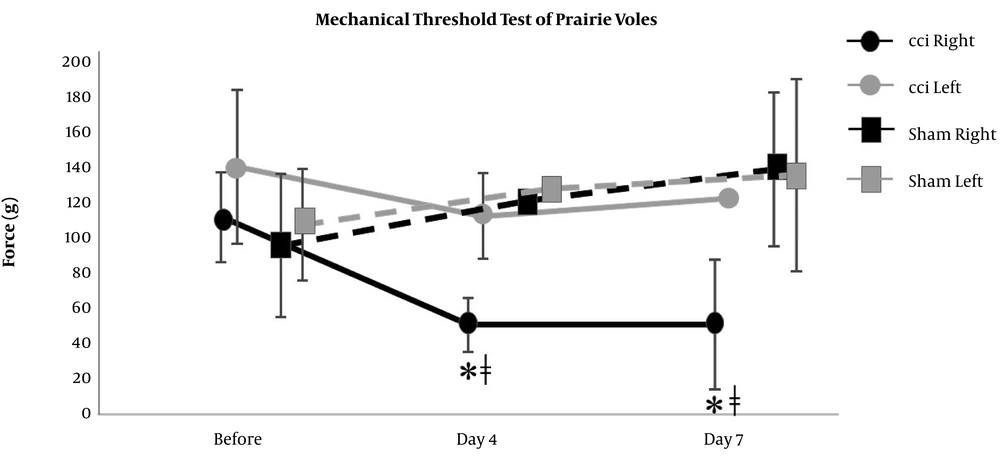
The right hind paw of CCI mice showed significant increases from the baseline level in the scores of nociceptive behavior on day 4 and day 7 (0 (0) at before, 0.8 (0.3) on day 4, and 0.8 (0.3) on day 7), which were significantly different from the scores of not only the left hind paw of CCI mice (0 (0) at before, 0 (0) on day 4, and 0 (0) on day 7) but also the hind paws of sham mice (0 (0) at before, 0.2 (0.4) on day 4, and 0.2 (0.4) on day 7) (Figure 2). Also, the right hind paw of CCI prairie voles showed significant increases from the baseline level in the scores of nociceptive behavior on day 4 and day 7 (0 (0) at before, 1.8 (0.4) on day 4, and 1.5 (0.5) on day 7), which were significantly different from the scores of not only the left hind paw of CCI prairie voles (0 (0) at before, 0 (0) on day 4, and 0 (0) on day 7) but also the hind paws of sham prairie voles (0 (0) at before, 0 (0) on day 4, and 0 (0) on day 7) (Figure 3). The right hind paw of CCI mice showed significant reductions from the baseline level in the mechanical threshold test results on day 4 and day 7 (110 (24) at before, 45 (37) on day 4, and 55 (35) on day 7), which were significantly different from the results of the hind paws of sham mice (102 (40) at before, 141 (47) on day 4, and 129(60) on day 7) (Figure 4). Besides, the right hind paw of CCI prairie voles showed significant reductions from the baseline level in the mechanical threshold test results on day 4 and day 7 (110 (25) at before, 50 (15) on day 4, and 50 (36) on day 7), which were significantly different from the results of not only the left hind paw of CCI prairie voles (138 (43) at before, 110 (24) on day 4, and 120 (0) on day 7) but also the hind paws of sham prairie voles (95 (40) at before, 120 (0) on day 4, and 138 (48) on day 7) (Figure 5). A gross movement of the trunk or extremities during the triple stimuli was mainly observed in the mechanical threshold test. That is, the results of the mechanical threshold test were consistent with the scoring of nociceptive behavior in CCI model animals.
5. Discussion
The present study demonstrated that the right hind paw of CCI animals showed significant increases in the scoring of nociceptive behavior on day 4 and day 7 and the right hind paw of CCI animals showed significant reductions in the mechanical threshold test on day 4 and day 7, which means that the results of the mechanical threshold test were consistent with the scoring of nociceptive behavior in CCI model animals.
Several behavioral tests have been devised to assess pain in rodent models, including the sensitivity of the paw evaluated through reflex reactions to heat or mechanical stimuli such as von Frey filaments (1-4, 11). In some animals, however, due to their high restless activity and responsiveness to humans, it is tough to give the moving animals consistent stimuli and get consistent, reliable reactions (3, 4). Sedation is a continuum that ranges from minimal to deep in a dose-response manner, which can be classified into levels: minimal, moderate, and deep sedation (5). In deep sedation, although subjects are asleep and unarousable, they show a purposeful response after repeated or painful stimulation. In the present study, deep sedation induced by inhalational anesthesia reduced animals’ high restless activity and responsiveness to human contact, thereby allowing us to stimulate them consistently by using a clip to get a reliable reaction.
5.1. Limitation
There are several limitations to the present study. Since painful stimulation is known to increase the arousal level, CCI-induced chronic pain might have influenced the isoflurane-induced sedation level in the present study. Thus, we should have monitored the sedation levels by using electroencephalography. The right hind paws of the CCI-administered side showed significant reductions from the baseline level in the mechanical threshold test, although the left hind paws of the non-CCI-administered side did not show any changes in the mechanical threshold test. We, therefore, postulate that CCI-induced chronic pain did not affect isoflurane-induced sedation levels in CCI animals.
5.2. Conclusions
The right hind paw of CCI animals showed significant increases from the baseline level in the scores of nociceptive behavior on day 4 and day 7 and the right hind paw of CCI animals showed significant reductions from the baseline level in the mechanical threshold test on day 4 and day 7, which means the results of the mechanical threshold test were consistent with the scoring of nociceptive behavior in CCI model animals and the method by using arterial clips under sedation was useful for the mechanical threshold test.