1. Background
Labor pain is probably the most severe pain a woman experiences throughout her life. Labor pain is one of the factors making the mothers not tolerate labor and request for elective cesarean section. This pain is caused mainly by the hypoxia of muscles, lactic acidosis, lower uterine segment distention, stretching of the ligaments, and pressure on the pelvis. The pain of the early first stage of labor comes from the dilatation of the lower segment of the uterus and cervix. The pain of the late first stage and second stage of labor arises from fetus descending in the birth canal and tearing of tissues in the vagina and perineum (1, 2).
Modern analgesia in labor began in 1947 by Doctor Simpson, who used ether and chloroform. There are some publications regarding spinal, lumbar, caudal epidural, pudendal, and paracervical blocks for obstetric reasons published between 1900 and 1930 (1-3). Nowadays, about 30% - 60% of women in Canada use epidural analgesia techniques to decrease labor pain (3). In the US, the use of epidural analgesia was tripled between 1981 and 2001, and 60% of women in large hospitals undergone this technique (4). Some complications may arise following the analgesia techniques, such as nausea, burning, itching, urinary retention, and even convulsion and respiratory depression (4-8).
On the other hand, some non-pharmacological methods are available for the management of labor pain, including massage, positioning, bathing, acupuncture, hypnosis, TENS, sterile water injection, etc. These non-pharmacological methods should be discussed when counseling with pregnant women (9). These methods are often simple, available, low-cost, and without side effects.
The intradermal injection of normal saline for pain relief was described in the 1970s. This method is easy and low-cost and does not require special experience. There are no known side effects for mother and fetus, as well (10-17). The method of cutaneous injection of sterile water for relieving labor pain comes from the gate control theory of Melzack and Wall on pain perception. This theory says that non-painful input closes nerve gates to other painful inputs and prevents pain sensation (18). The cutaneous injection of distilled water in parturient women is a new pain stimulus that changes the perception of labor pain.
Some studies have shown that intradermal sterile water injection is effective in reducing labor pain (18-20), and it may decrease the rate of cesarean section (21). In a multicenter study in Australia on 1,866 women in labor aged ≥ 18-years-old with singleton cephalic presentation, gestational age ≥ 37, and VAS score ≥ 7, the effect of sterile water injection was assessed on the control of labor pain. Thus, 0.1 - 0.3 cc sterile water or normal saline (as placebo) was injected intradermally at four sacral points. They showed a significant reduction in the cesarean section rate in the sterile water injection group (22). In another study on 100 parturient women in India, 50 women were injected with 4 cc sterile water and 50 women with 4 cc normal saline intradermally on the lumbosacral area. The VAS score, labor progression, and fetal outcomes were assessed 10, 45, and 90 min after the intervention. There was a significant difference in the reduction of pain scores between the sterile water injection group and normal saline injection group (P < 0.05). There was a significant pain reduction in the sterile water injection group but not in the normal saline injection group. It was concluded that the intracutaneous sterile water injection over the sacrum was a simple and effective method to control labor pain (23). A Cochrane study of labor pain control, the effects of intradermal and subdermal sterile water injections were compared with a control group. The results of seven studies with 766 samples were analyzed. Four studies assessed intradermal injection, two studies assessed subdermal injection, and one study assessed both intradermal and subdermal injections of sterile water. Sterile water injection reduced labor pain by at least 50%, while the placebo reduced labor pain by 20% (16). One RCT also used the intradermal sterile water injection for the control of acute low-back pain. The sample included 41 women and 27 men, aged 18 - 55 years, with a VAS score of ≥ 5. Intradermal sterile water injection was used for 34 cases and subdermal sterile water injection for the other 34 cases. The VAS scores were recorded 10 min, 45 min, 90 min, and 24 h after injections. The pain score was 2.9 ± 2.21 in the intradermal group and 0.9 ± 2.22 in the subdermal group (P < 0.05). They concluded that the subdermal injection was more effective for short-term relief (24).
2. Objectives
As there are different results regarding pain control by intradermal and subdermal injections, in a randomized, double-blind placebo-controlled clinical trial, we compared the effects of intradermal and subdermal sterile water injections on active labor pain. The main clinical goal of this study was to compare these two methods with each other and with a placebo. We tried to determine if intradermal and subdermal injection methods are effective in labor pain control, and which one is more effective?
3. Methods
This randomized, double-blind placebo-controlled clinical trial study was conducted at a university maternity hospital (Shahid Akbarabadi Hospital). The study sample included 121 term nulliparous pregnant women in the second stage of labor (dilatation 4 cm or more) who met the inclusion criteria. The inclusion criteria were singleton nulliparous pregnant women candidate for vaginal delivery at the gestational age ≥ 37 weeks with cephalic presentation and 4 cm dilatation (or more) and a VAS pain score ≥ 5.
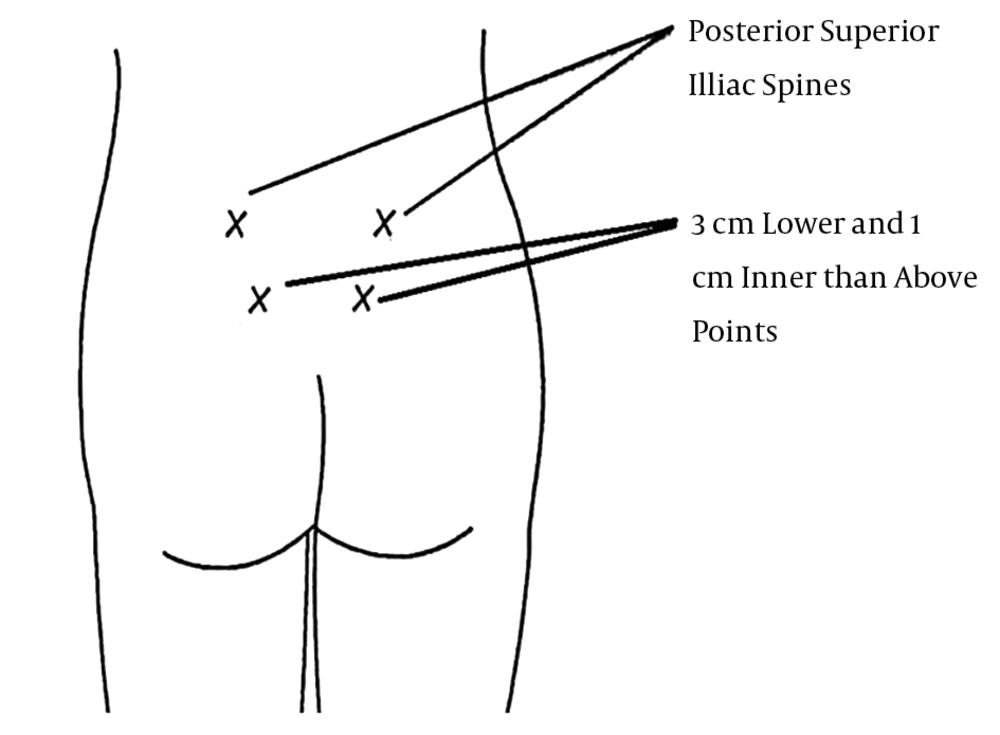
The study initiated after approval of the Ethics Committee of the University. All nulliparous pregnant women meeting the inclusion criteria who signed informed consent forms were enrolled for our research. The pregnant women were assigned to one of the three groups by block randomization. Group one was injected subdermally with 0.5 cc sterile water (distilled water, Plastic ampoule 5 cc, Aqua Pura, Samen Co., Iran) using insulin needles at four sacral points (Figure 1). Group two was injected intradermally with 0.5 cc sterile water using insulin needles at four sacral points. Group three was only contacted with insulin needles at the mentioned points (control group). The intervention was performed by a single resident of Obstetrics and Gynecology. The sites of injections and intervention are shown in Figure 1 (0.5 cc sterile water on each posterior superior iliac spine and 0.5 cc three centimeters lower and one centimeter inner than the above points).
The VAS score was determined before the intervention and 10, 30, 60, and 90 min after the intervention. For double-blinding of the study, the VAS scores before the intervention were measured by the resident of Gynecology, and the VAS scores after the intervention were measured by a labor midwife. In addition, each intervention was performed in separate Labor Delivery Rooms (LDR). Thus, the patients were not aware of the type of intervention (subdermal, intradermal, or only needle contact to skin).
We did not use any analgesics within 90 min after the intervention. This was because most of the parturient women were near delivery and we intended to assess the side effects of our intervention. Moreover, before entering the study and signing informed consent forms, we consulted with all of the parturient women and described that we would only use a single injection in this study. However, if a mother requested for another analgesic method, we excluded her from the study and enrolled another parturient.
3.1. Statistical Analysis
Based on a previous study (16) that showed the labor pain reduction of 50% in the sterile water injection group and 20% in the placebo group, P1 = 0.50, and P2 = 0.20, the sample size was 120 (n = 40 for each intervention group).
The data analysis was done using SPSS 22. Quantitative variables were expressed as mean and standard deviation and qualitative variables as percentages. Quantitative variables were compared with the t-test, and the Mann-Whitney U test was used for non-normal distributed variables. The qualitative variables were compared using chi-square or Fisher’s exact test. The correlation between quantitative variables was investigated using Pearson correlation coefficient and Spearman rank correlation.
4. Results
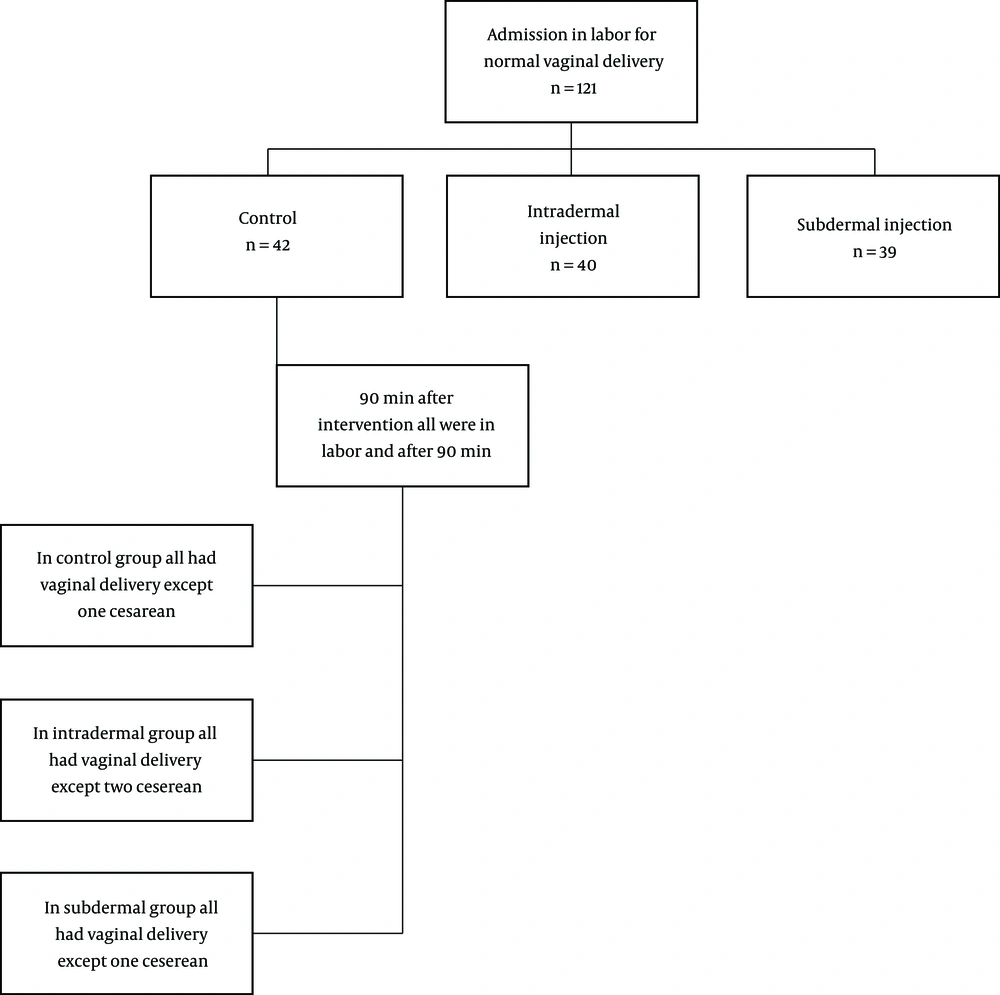
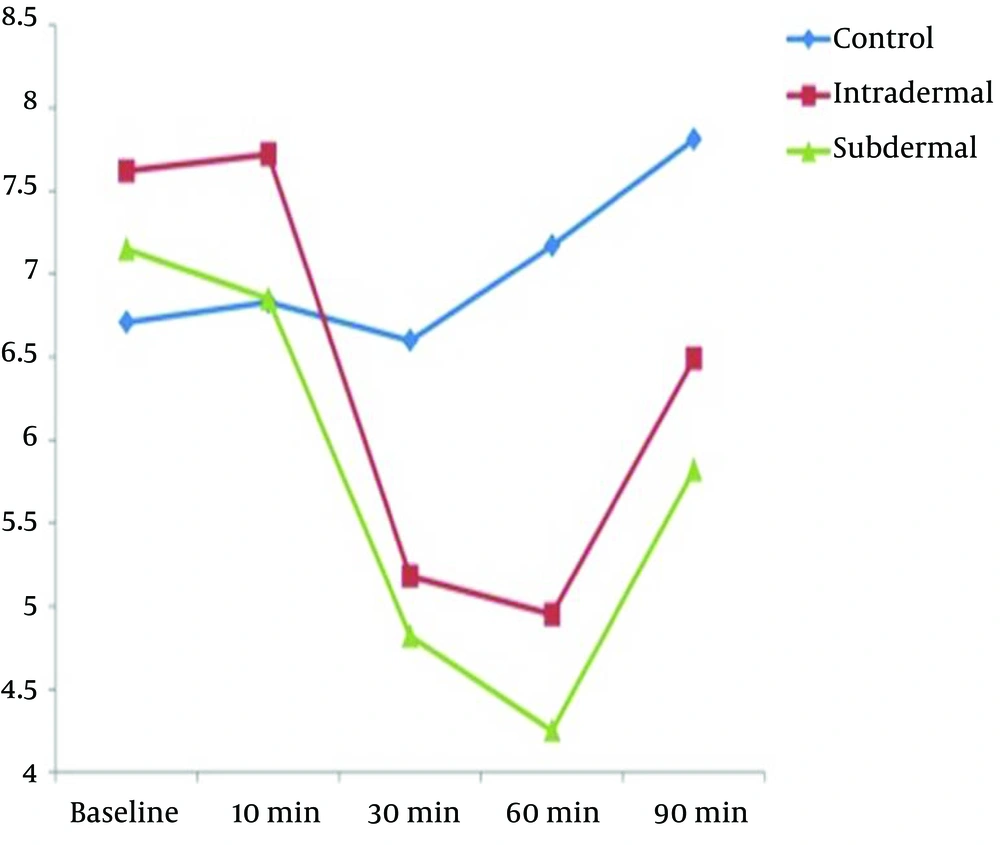
In this double-blind clinical trial, 121 nulliparous pregnant women meeting the inclusion criteria were assessed in three groups (Figure 2). The three groups did not have significant differences in demographic variables (Table 1). The VAS score had no significant difference between the three groups before the intervention (P = 0.076) (Table 2 and Figure 3). The mean VAS score 10 min after the intervention had no significant difference between the groups (P = 0.061) (Table 2). The mean VAS score 30, 60, and 90 min after the intervention had significant differences between the three groups (P = 0.001) (Table 2). The mean percentage of pain reduction after 90 min was 25% in intradermal injection and 30% in subdermal injection, which showed no significant difference. There was no significant difference between the intradermal and subdermal injection results, but the pain score was significantly lower than that of the control group (P = 0.006). During the first 90 min after the intervention, all of the participants continued labor without any problem or request for more analgesia. However, 90 min after the intervention, we had one cesarean delivery due to the arrest of dilatation in the control group, one cesarean delivery due to variable deceleration and one due to failure to descent in the intradermal injection group, and one cesarean delivery due to the arrest of descent due to short umbilical cord in the subdermal injection group. All of the mothers delivered within five hours after the intervention. In one case from the placebo group, the mother requested more analgesia after 90 min of intervention; thus, we excluded her and enrolled another mother (Figure 2).
| Characteristics | Control (N = 42) | Intradermal (N = 39) | Subdermal (N = 40) | P Value |
|---|---|---|---|---|
| Mean age | 23.62 ± 5.02 | 22.28 ± 4.96 | 24.78 ± 4.97 | 0.089 |
| Education level | 0.505 | |||
| Illiterate | 0 (0.0) | 1 (2) | 0 (0.0) | |
| Elementary | 8 (19) | 6 (15) | 9 (22) | |
| High school | 14 (33) | 11 (28) | 14 (35) | |
| Diploma | 16 (38) | 18 (46) | 10 (25) | |
| Academic | 4 (9) | 3 (7) | 7 (17.5) | |
| Job status | 0.43 | |||
| Housewife | 37 (88) | 36 (92) | 34 (85) | |
| Worker | 2 (4) | 0 (0.0) | 0 (0) | |
| Employee | 2 (4) | 1 (2) | 3 (7.5) | |
| Self-employed | 1 (2) | 2 (5) | 3 (7) | |
| Dilation before intervention | 6.56 ± 1.42 | 6.31 ± 0.52 | 6.20 ± 0.46 | 0.313 |
Demographic Data of Patients in Three Groupsa
| Characteristics | Control (N = 42) | Intradermal (N = 40) | Subdermal (N = 39) | P Value |
|---|---|---|---|---|
| Before intervention | 6.71 ± 1.79 | 7.62 ±1.77 | 7.15 ± 1.73 | 0.076 |
| 10 min after intervention | 6.83 ± 1.72 | 7.72 ± 1.68 | 6.85± 1.9 | 0.061 |
| 30 min after intervention | 6.60 ± 1.91 | 5.18 ± 1.94 | 4.82 ± 1.93 | 0.001 |
| 60 min after intervention | 7.17 ± 1.88 | 4.95 ± 1.83 | 4.25± 2.13 | 0.001 |
| 90 min after intervention | 7.81 ±1.68 | 6.49 ± 1.99 | 5.82 ± 2.74 | 0.001 |
Comparison of Pain Score Before and After Intervention in Three Groupsa
The mean APGAR score in normal vaginal deliveries was 8 in the control group, 8.5 ± 0.25 in the intradermal injection group, and 8.5 ± 0.5 in the subdermal injection group. The APGAR score did not have significant differences between the three groups.
5. Discussion
In this clinical trial of the effect of intradermal and subdermal sterile water injection on active labor pain, there were no significant differences between the results of the two groups, but these groups were significantly different from the control group in labor pain relief (P = 0.001).
One of the factors encouraging pregnant women to choose elective cesarean section delivery is the experience of labor pain associated with severe lumbar pain. Various methods and tools are used to mitigate the pain during labor, but each of them has some limitations and complications. Recently, intradermal and subdermal injection of distilled water has been recognized to be effective in improving this pain. However, the question is which of the methods (intradermal or subdermal injection) has a greater impact on pain improvement and patient satisfaction. This study investigated the trend of variations in the patients’ pain scores within 90 min after the intervention. We showed that the use of distilled water injection in both methods could lead to a significant reduction in labor pain during 90 min after the intervention. However, this effect on the pain severity was not seen during the first 10 min of intervention.
Given the availability and cost-effectiveness of distilled water, this protocol can replace analgesic drugs or even aggressive methods. In a survey, 168 healthy term women with labor pain were randomized into dry injection (placebo group) and intradermal sterile water injection. The pain scores were assessed by the VAS at 10, 30, 60, 120, and 180 min after the intervention. The mean pain scores 30 min after injections were lower in the sterile water injection group (P < 0.01). The need for epidural analgesia, duration of delivery, mode of delivery, and APGAR scores were similar in both groups (25). Another study showed no difference in the pain reduction percentage between the two groups receiving distilled water by intradermal and subdermal injection methods (13), which was quite similar to our study results. Interestingly, in the current study, the duration of analgesia caused by distilled water lasted up to 90 min after the injection. In a study, the pain score reduction following distilled water injection was approximately 50% - 60% versus 20% - 25% in the placebo group (14), which is similar to this study in terms of the difference between distilled water injection and the placebo. In a meta-analysis, the incidence of cesarean section was 4.6% in the distilled water injection group and 9.9% in the placebo group. Thus, in the cases with labor pain, the tendency to change the delivery approach to the cesarean section significantly reduced in the distilled water injection group (15, 16). In our study, the rate of cesarean sections did not have a significant difference between the control and sterile water injection groups. In another study, the mean pain scores 30 min after injection were significantly lower in the distilled water group than in the placebo group (17). In another study, the severity of pain in the distilled water injection group was significantly lower than that in the placebo group at all measured times (24). Although it was not our study objective, we showed that the frequency of distilled water injection had a direct relationship with pain severity reduction. In a study, there was more pain reduction in the group that received distilled water four times than the other group that received distilled water only once, but there were no differences between intradermal and subdermal injection in the use of another analgesic agent, delivery method, and maternal satisfaction (21).
Although sterile water in our study and some other studies was effective in the control of labor pain, its use is not common, possibly due to the low knowledge of midwives and obstetrician. For example, sterile water injection is uncommon in the UK. Although midwives were interested in using these procedures, 82% of the midwives did not use sterile water injection in practice and 69% would consider learning the procedure. The restrictive factors in using sterile water injection in the UK were the lack of available guidelines from the National Institute of Health and Care in the UK and the lack of information and training in midwives (26). In an online invitation from the Australian College of Midwives, 970 midwives completed a questionnaire on sterile water injection. It was shown that 42.5% of the midwives were the current users of sterile water injection, 86% answered that they would consider using sterile water injection, and 90% were interested in obtaining further information about sterile water injection (27). In a meta-analysis of sterile water injection for labor pain, both intradermal and subdermal injections were effective for pain relief but the subcutaneous technique was less painful than the intradermal technique. The effect seemed to be related to the amount of sterile water in each injection and the number of injections. In general, four injection sites are recommended as the injections can be repeated without adverse effects for the mother and fetus (28).
Because of the side effects, costs, and mothers’ fear of using invasive methods for relieving labor pain, it is suggested that non-pharmacological methods like sterile water injection be discussed when counseling with mothers during pregnancy (9).
5.1. Conclusions
It can be concluded that the lumbosacral injection of distilled water by either intradermal or subdermal method was associated with a significant reduction in pain severity during labor, but there was no difference between the intradermal and subdermal methods in terms of relieving the labor pain. As this is a safe, effective, and low-cost method, it is proposed to increase the knowledge of midwives and obstetricians about this method.
5.2. Study Limitations
After the intervention, a mother was excluded from the study as she requested for continuous epidural or other analgesic methods.
For Future: We suggest that future studies be conducted with higher sample sizes and more frequency of sterile water injection for labor pain control.