1. Background
Chronic myeloid leukemia (CML) is a clonal disorder in which the hematopoietic stem cells (HSCs) or multipotent progenitor cells transform into leukemic cells. The annual incidence of CML is about 1 to 2 cases per 100,000 individuals (1). It is noted that in more than 90% of patients, the major characteristic of the disease is the presence of the BCR-ABL hybrid oncogene (2). BCR-ABL is an active kind of tyrosine kinase that plays fundamental biological roles, including HSCs transformation, and proliferation or expansion of leukemic stem cells (LSCs) (2, 3). For years, Arsenic, radiotherapy, busulfan, and, later, Interferon-alpha were the main first well-documented line of treatment for CML patients. Although those treatments could increase the survival rates of approximately one-third of patients, they were replaced with new drugs due to inefficiency and several side effects (3).
Soon after, the development of targeted therapies revolutionized the CML treatment options. The Tyrosine kinase inhibitors (TKIs) were introduced as therapeutic agents to overcome the progression of CML. Although the TKIs considerably enhance control of chronic phases of CML, such treatments are not curative. For example, increasing evidence demonstrated that some patients show resistance to imatinib as the first-generation targeted therapy with TKIs. In this case, the 2-phenylaminopyrimidine compound that inhibits the BCR-ABL oncoprotein in CML cells gives rise to quiescent leukemia cells responsible for disease relapse (4). In this regard, therapies that target genes or pathways involved in the survival/self-renewal of LSCs could be novel treatment options for reducing aberrant self-renewal of LSCs and drug resistance (4).
The TGF-β signaling pathway is one of the fundamental pathways with over 30 members, including 3 TGF-β, 4 Activins, and bone morphogenetic proteins (BMPs). It has a vital role in the growth/differentiation balance in hematopoietic cells (5). TGF-β has a dual role in cancer cells, as a strong tumor inhibitor in the earlier stages of CML and as a tumor promoter in the progressive phases (6, 7). Besides, in order to understand the function of CML stem cells, TGF-β could open up new opportunities for developing novel drugs (8).
Smads are known as the number of effector proteins directly linked to TGF-β signaling events. These proteins are classified into three types based on their structural and functional characteristics: (1) the receptor-regulated Smads (R-Smads), (2) the common-mediator Smads (co-Smads), and (3) the antagonistic or inhibitory Smads (I-Smads). Among all, Smad1-3, 5, and 8 influence the activation of the kinase domain of R1 and undergo phosphorylation and activation. Meanwhile, Smad4 forms complex with R-Smad and translocate into the nucleus, where they act as transcription factors (7).
MicroRNAs (miRNAs) are small non-coding RNAs with 22 nucleotides with extensive roles in the regulation of gene expression and target destabilization (9, 10). Depending on the specific target gene, miRNAs contribute to controlling several biological processes, cellular proliferation, tissue differentiation, signal translocation, stem cell maintenance, and leukemogenesis (11). MiR-144 acts as an oncogene or tumor suppressor by targeting different molecules. It is one of the molecules with a crucial role in modulating the TGF-β pathway and, therefore, could open a potential therapeutic window in controlling the CML disease.
In leukemic stem cells, over-expression of the TGF-β signaling pathway is one of the main issues, leading to chemo/drug resistance. Evidence suggest a significant connection between TGF-β/Smad pathways with hematological malignancies (12). For example, dysregulated Smad4 and TGF-β signaling were noted in several types of cancers (13).
2. Objectives
Despite different clinical studies, unfortunately, we are far from finding precise treatment for CML patients. Accordingly, it is critical to explore the fundamental molecular pathways involved in the CML microenvironment. Taken together, this paper aimed to investigate the effect of overexpressed miR-144 on Smad4 and TGF-βR2 expression in CML CD34+ cells.
3. Methods
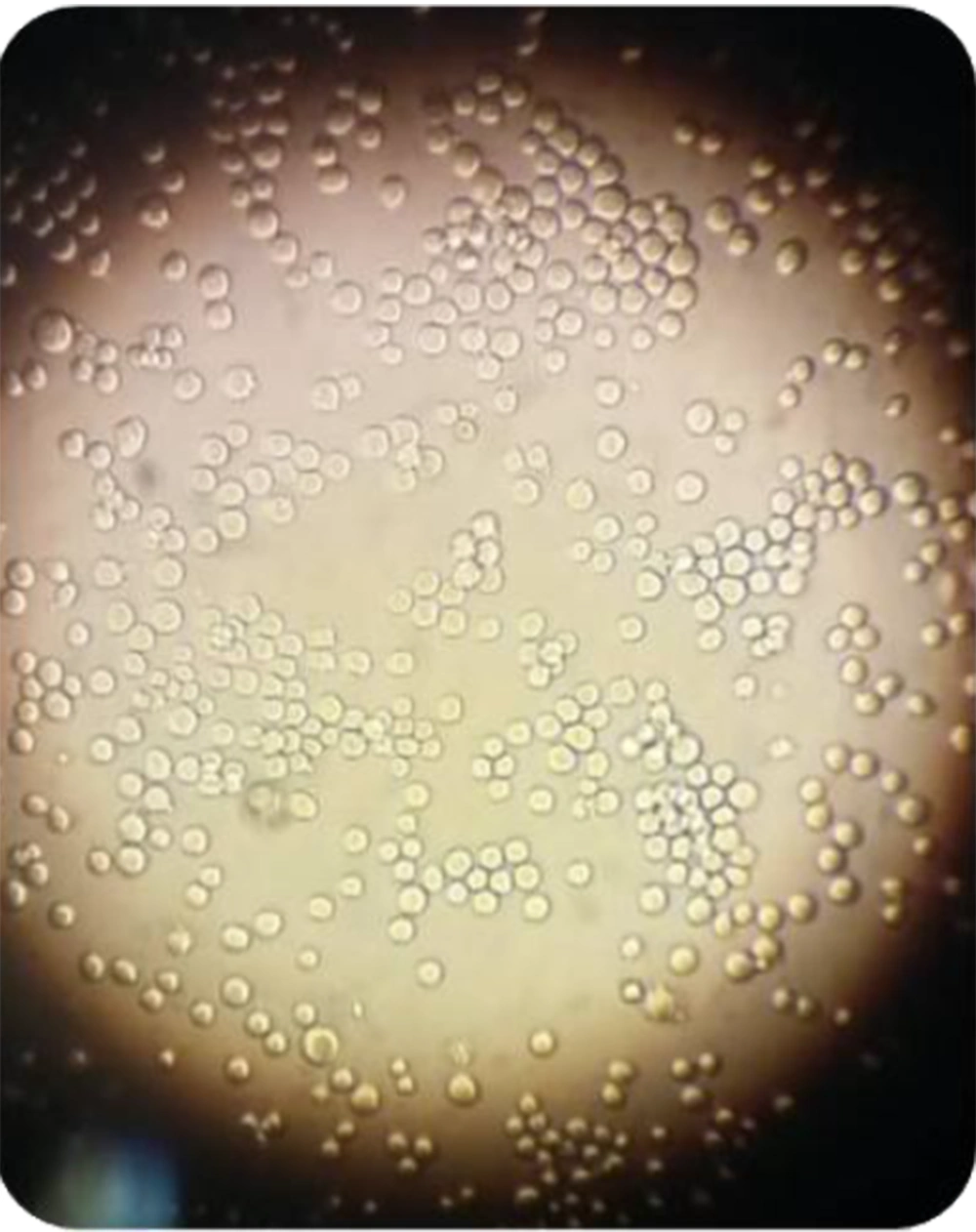
3.1. Isolation and Primary Culture of CML CD34+ Cells
CD34+ cells were isolated from the bone marrow of three CML patients with no history of treatment. Written informed consent was obtained from all patients. The cells were cultured in Stemspan H3000 medium (Cat#100-0073) containing a high concentration of growth factors (eg, Flt-3, SCF, and TPO).
3.2. CML CD34+ Cells Characterization
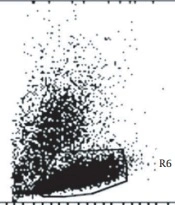
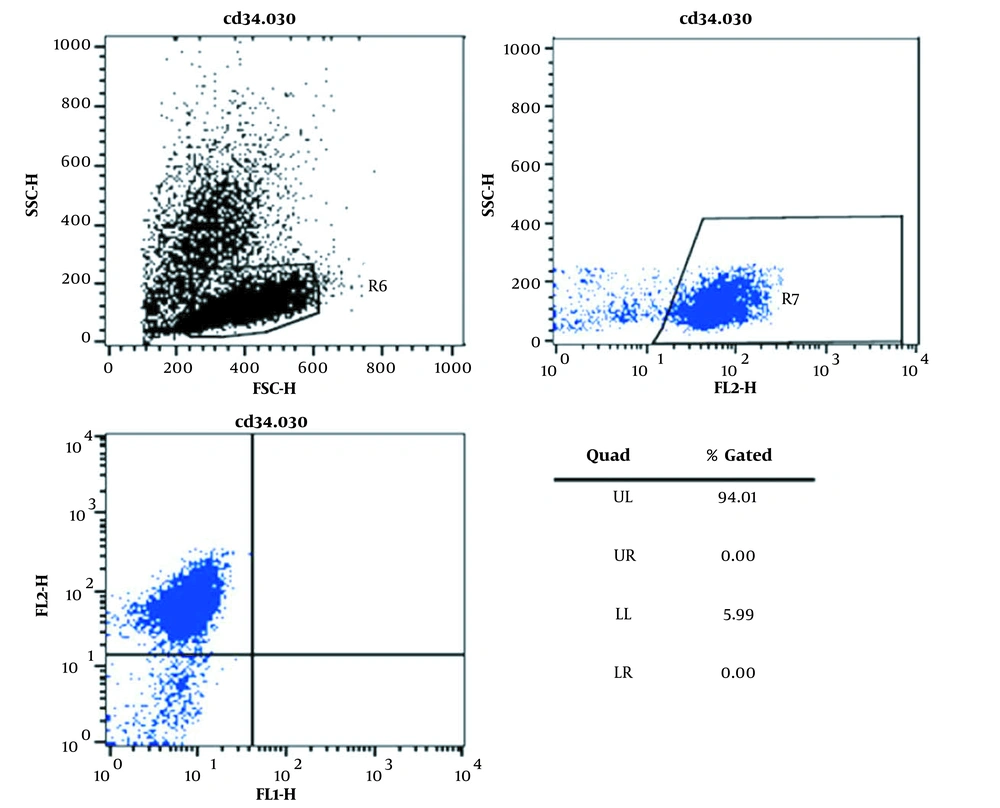
For phenotypic characterization of the isolated cells, flow cytometry (Becton–Dickinson, San Jose, CA) was performed. Briefly, 1 × 105 of isolated cells (passages 3 - 5) were studied for the expression of the CD34+ markers of the hematopoietic stem cells (14).
3.3. The CML CD34+ Cells Transfection
Total number of 2 × 104 cells were cultured in 24 well culture plates containing low glucose DMEM medium (Gibco) supplemented with 10% FBS (Gibco) and were maintained in an incubator with a 5% CO2 environment. When the cells reached 80 - 90% confluency, the cells were transfected with miR-144 using Lipofectamine reagent (Sigma) according to standard protocols (15).
3.4. Reverse Transcriptase-polymerase Chain Reaction
The harvested cells were suspended in TRIzol reagent (Invitrogen, CA, USA), and the total RNA was extracted according to the manufacturer's recommended procedures. To remove the genomic DNA contamination and gain high purity, the extracted RNA was treated by RNase-free DNase I (Invitrogen, CA, USA) based on the manufacturer's instructions. The purity of the extracted RNA was examined by gel electrophoresis. Then, cDNA was synthesized using miRNA 1st-strand cDNA synthesis (Parsgenome) following the manufacturer's guidelines. The expression levels of miR-144, Smad4, and TGFβR2 genes were evaluated by reverse transcriptase-polymerase chain reaction (RT-PCR) using SYBR Green PCR master mix (Bio Fact, Korea) and specific primers following standard conditions. mRNA levels were quantified by comparison with GAPDH expression (as the standard control) using the 2-ΔΔCt method. The primers were designed using Gene Runner v.3.05 and Oligo version 7.
3.5. Evaluation of Cell Proliferation
For assessment of cell viability, 1 × 105 transfected or non-transfected cells (control group) were cultured in 24-wells plates and counted for 2 consecutive days using a neobar lam.
3.6. Statistical Analysis
Data analysis was administered using the IBM SPSS Statistics for Windows, version 24.0 (Armonk, NY, USA), and graphs were depicted by GraphPad Prism 8 software (Inc., USA). The difference between groups was evaluated using the t-test. Statistical significance was considered when the P-value < 0.05.
4. Results
4.1. Characterization of CML CD34+ Cells
As shown in Figure 1, the isolated cells appeared round-shape and were similar to the morphology of hematopoietic stem cells. Moreover, flow cytometry results showed that more than 90% of the isolated cells expressed the CD34 marker (Figure 2).
4.2. Evaluation of miR-144 Expression Level
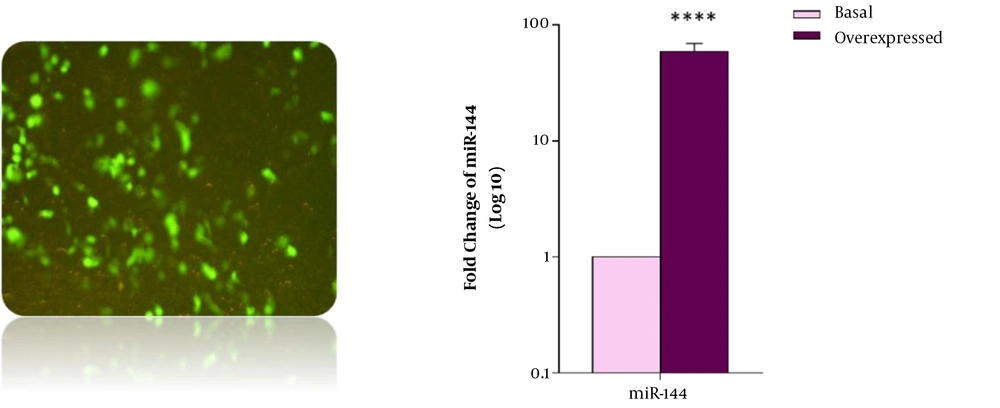
Forty-eight hours after transfection of CD34+ CML cells with miR-144 precursor sequence, assessment of miRNA expression levels was done by real-time PCR in both transfected and non-transfected cells (as the control group). The data showed a significant increase in miR-144 expression in transfected cells compared with the control group.
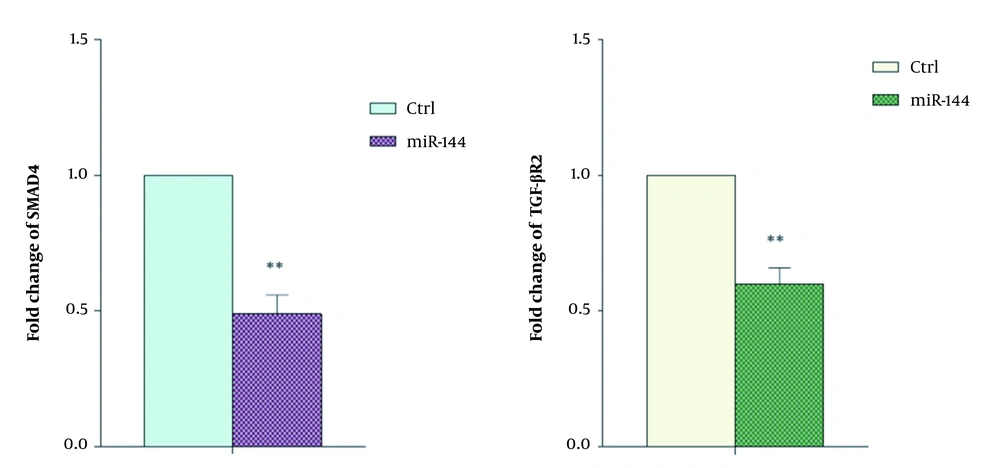
4.3. Evaluation of SMAD4 and TGFβR2 Expression Levels
In order to investigate the effect of overexpressed miR-144 on the expression of SMAD4 and TGFβR2 genes, the real-time RT-PCR technique was performed. The results revealed a significant decrease in Smad4 and TGFβR2 expression in the transfected cells compared with control cells (Figures 3 and 4).
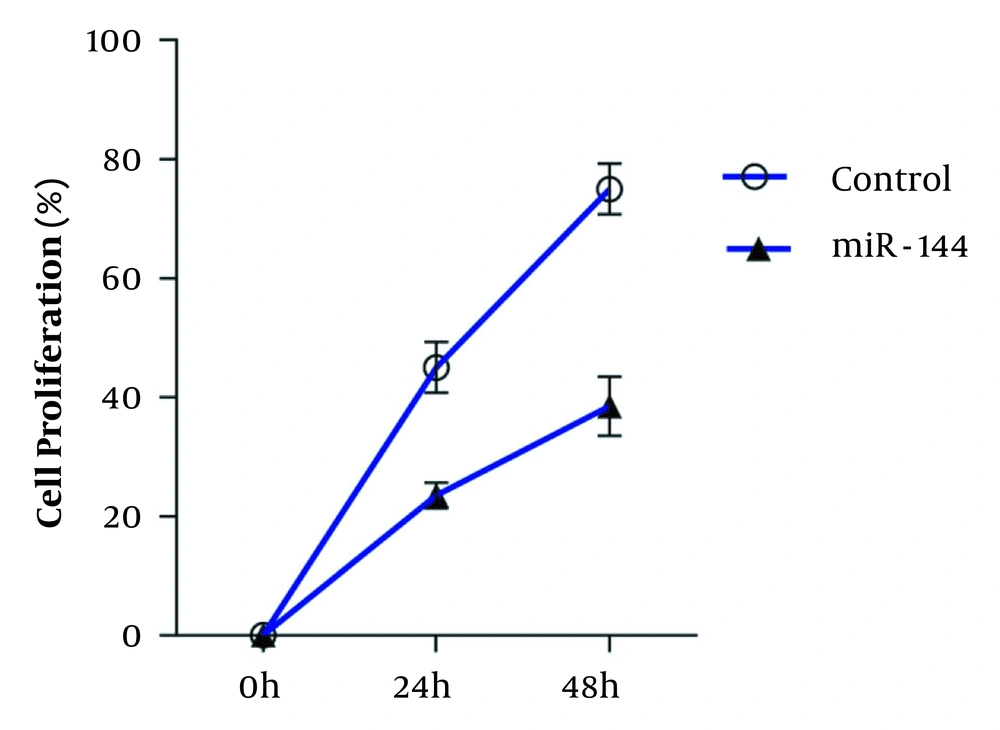
4.4. Evaluation of Cell Proliferation
Following overexpression of miR-144, cell proliferation rates were compared with control cells. The results demonstrated an increase in miR-144 expression level, which was associated with a decrease in Smad4 and TGFβR2 expression levels, accompanied by reduced cell proliferation in a time-dependent manner (Figure 5).
5. Discussion
Translocation of Abelson murine leukemia viral oncogene homolog (ABL) and breakpoint cluster region (BCR) between chromosomes 9 and 22 are the most common representatives of CML patients. The role of BCR-ABL kinase activity in these patients provided the rationale for developing various generations of BCR-ABL kinase inhibitors in targeted therapy of CML. Despite their relative effectiveness and the successful therapies in the treatment of CML patients, increasing evidence indicate the recurrence of the disease and emergence of drug-resistant cells, even after complete remission.
Studies revealed that TGF-β signaling mainly affects the CML stem cells. In this subject, the experiment results of Naka and colleagues showed that inhibition of endogenous TGF-β signaling pathway in CML-LIC cells migth be a promising strategy to suppress the functions of these cells (16). Significantly, the endogenous TGF-β signaling pathway could be considered to design novel therapies for CML disease.
Moller et al. indicated that up-regulation of the TGF-β pathway by BCR-ABL maybe one of the mechanisms involved in hematopoietic progenitor cell deformation. They proposed that TGF-β could play a critical role in maintaining the malignant precursor population and, partly, was responsible for resistance to BCR-ABL therapy, which has been observed in some CML patients (8). CML stem cells' resistance to BCR-ABL kinase inhibitors is capable of initiating the leukemic colonial hierarchy.
Smad 4 is essential for the formation of heterologous complexes with Smad2 and Smad3, as well as for its transfer to the nucleus. Its low expression could be another potential reason for inhibiting this tumor suppressor pathway (17). Shokeen et al. tried to find more direct links to changes in TGF-β/Smad signaling pathways and CML patients. They reported enhanced expression levels of the TGF-β/Smad pathway by BCR-ABL in CML cell lines (12).
Identifying the molecular pathways related to the survival/self-renewal of CML stem cells is of fundamental importance for the effective treatment of CML. Accordingly, increased expression levels of the TGF-β pathway members in the advanced stages and Imatinib-refractory cases of the CML disease can be an important clue for developing precision medicine approaches. These findings could help explain why TKI therapy cannot thoroughly eradicate the CML disease (18, 19).
miRNAs can act as oncogenes or tumor suppressors, so understanding and modulating their biology and activity could open new opportunities for leukemia treatments. miRNA involvement in leukemia disorder has created an understanding of the different levels of its complexity. Targeting specific messaging pathways in leukemia stem cell survival characteristics has not been well elucidated. Therefore, providing a rational solution is essential for managing drug-resistant CML patients. Likewise, TGF-β messaging should be considered for designing novel therapies for CML. Thus, to the best of our learning, we evaluated the effects of up-regulated miR-144 in the TGF-β signaling pathway of CML patients.
Although BCR-ABL facilitates the leukemic transformation by increasing oncogenic miRNAs expression and decreasing the expression of tumor suppressor miRNAs, there is compelling evidence for the misplaced expression of a miRNA independent of BCR-ABL activity. In this regard, Agirre and colleagues identified a profile of aberrant miRNAs expression in CD34+ cells, and an analysis of 157 miRNAs indicated that miR-10a, miR-150, and miR-151 were negatively regulated in CD34+ cells of CML patients (except for miR-96) (20). On the other hand, available studies showed the regulatory role of miR-144 in both TGFβ and Smad4 signaling (21, 22).
So, we investigated whether targeting Smad4 and TGFβR2 by upregulation of miR-144 could lead to decreased TGF-β signaling pathway activity and CD34+ cell proliferation. Our real-time RT-PCR results demonstrated the simultaneous downregulation of Smad4 and TGFβR2 in CD34+ cells transfected with oligonucleotide miR-144. Therefore, these findings indicated the simultaneous targeting of the SMAD4 and TGF-β R2 expressions by miR-144 alongside an efficient expression system. This finding suggests that miR-144 can be used to target TGF-β signaling in CML cases with elevated TGF-β as a precision medicine strategy.
Another intriguing result of the current study is reduced cell proliferation by miR-144 overexpression in CML CD34+ cells. These findings are consistent with the proliferative function of the TGF-β signaling pathway that can enhance the cell cycle via its up-regulation (17). Importantly, CML CD34+ cells are the same leukemia stem cells that are not affected by the Imatinib treatment process and cause recurrences. Therefore, the current approach, which directly targets the key members involved in the proliferation/survival of stem cells, is of great clinical importance. Further pre-clinical assessment of the miRNA function will help to develop therapeutic methods intended to eradicate CML disorder. Finally, it suggests that the other influential miRNAs involved in the CML CD34+ cell behavior should be profiled. Indeed, identifying the more molecular targets of miR-144 involved in the survival of CML CD34+ cells could contribute to a better understanding of miR-144 function. Moreover, evaluating the functional roles of overexpressed miR-144 in the mouse model of CML disorder proposes more investigations. Our study highlights the opportunities in the application of miR-144 as a viable TGF-β targeting agent and diagnostic biomarker in CML. These miR-144 characteristics can be exploited in a precision medicine approach to identify TGF-β sensitive CMLs based on miR-144 expression and/or target TGF-β/Smad pathway, which in turn can reduce CML cell proliferation.
5.1. Conclusions
The expression alterations of miRNAs are common characteristics of hematopoietic malignancies like leukemia. Moreover, the TGF-β signaling pathway acts as a tumor promoter in the late stage of the disease. So, it can be an attractive target for CML treatment. Collectively, the finding of the present study demonstrated that miR-144 overexpression induced a significant reduction of CD34+ cell proliferation via down-regulation of Smad4 and TGF-β R2, as critical elements of the cell cycle. Addressing Smad4 and TGF-β R2 by miR-144 could play a fundamental role in preventing the development of CML disease. Moreover, the results of the current experiment indicated that overexpressed miR-144 could induce apoptosis in CML CD34+ cells, as potential reservoirs of disease recurrence, by aiming Smad4 and TGF-β R2. It is emphasized that CML CD34+ cells are drug-resistant stem cells and cause disease recurrence after chemotherapy. In this regard, the current study could modulate the TGF-β signaling pathway and motivate apoptosis by inducing the expression of a key miRNA.