1. Background
Androgenetic alopecia (AGA), or male-pattern hair loss (MPHL), is the most common form of hair loss occurring in 80% of men aged 80 years or older (1). The AGA is an androgen-dependent disorder, and the miniaturization of scalp hair follicles results in decreased hair density, thin fibers, and severe forms that cause baldness (2, 3). Dihydrotestosterone (DHT) is the primary specific androgen hormone involved in AGA. In the DHT biosynthesis pathway, an integral membrane enzyme, 5 alpha-reductase (5AR), plays a regulatory role by reducing testosterone to DHT.
The 5AR family is related to androgen-dependent disorders and consists of two isozymes, 5 alpha-reductase type 1 (5AR1) and 5 alpha-reductase type 2 (5AR2) (4, 5). The 5AR1 and 5AR2 are nicotinamide adenine dinucleotide phosphate-dependent enzymes and share a low sequence identity of 47%. These two isozymes are encoded by SRD5A1 and SRD5A2 genes, located in different chromosomal locations. The 5AR1 is located on the 5p15.31 chromosome; however, 5AR2 is located on chromosome 2P23.1 in the human genome (6).
Moreover, the tissue expression patterns of 5AR1 and 5AR2 are different. Type 1 is expressed in nongenital skin, fetal scalp, and liver; nevertheless, type 2 is predominantly expressed in male genital tissues, prostate, and seminal vesicles. The 5AR2 is responsible for virilizing the external male genitalia during puberty, benign prostatic hyperplasia (BPH), and prostate adenocarcinoma tissues (7-9). Since it has been reported that DHT-related disorders include AGA, BPH, and prostate cancer, which can be treated by lowering DHT levels, 5AR is identified as an efficient drug target (10).
The 5AR inhibitors are applied in restoring hair loss and regenerating miniaturized hair. Hair restoration is defined by the change in terminal hair count and width. Nonsurgical hair restoration options ranging from drug therapy to laser treatments are cheaper and do not carry risks associated with surgical hairline restoration, including hair transplantation (11). In this regard, two potent synthetic drugs, including finasteride and dutasteride, have been established to inhibit 5AR1 and 5AR2. Finasteride, a synthetic azo-steroid, is a more potent 5AR2 inhibitor than 5AR1, and dutasteride, widely used as AGA or BPH drug, inhibits both 5AR1 and 5AR2 (12).
According to the research conducted on the above-mentioned two drugs, it has been detected that finasteride can reduce the DHT level in serum by 70%, and the dutasteride inhibition effect showed a reducing amount of DHT level up to 90% in serum (13). Although finasteride and dutasteride performed well as efficient 5AR1 inhibitors and have been approved by the United States Food and Drug Administration (FDA), there have been more concerns about these two drugs’ long-lasting side effects occurring in therapy within the last few years. Recent research has shown that finasteride and dutasteride have several sexual adverse effects, including libido reduction, dysfunction of erectile, ejaculation disorders, and gynecomastia (14, 15). Due to the safety concerns of these drugs, the detection of new, safe, and more effective 5AR inhibitors has become a new medication approach in the drug industry. As it is evidenced that 5AR1 is primarily expressed in the scalp, the inhibition of 5AR1 is the primary approach for AGA medication (16).
2. Objectives
Multiple traditional herbals are suggested as potent drugs to prevent this enzyme from working normally. The current study provided a library of herbal components as natural inhibitors and evaluated 5AR1 through homology modeling and molecular docking techniques. The current study library includes Cedrus and the henna tree (Lawsonia inermis), widely used in home remedy medications as topical therapy on the scalp. In various studies, other compounds mentioned as effective 5AR1 inhibitors that suggested stimulating hair regrowth and strengthening hair follicles are saw palmetto (Serenoa repens) and green tea (Camellia sinensis). Another highly recommended compound is vitamin B2, also known as riboflavin. Moreover, two approved drugs, finasteride, and dutasteride, were chosen as positive controls to be compared with the aforementioned natural compounds (17-21).
3. Methods
3.1. Molecular Modeling
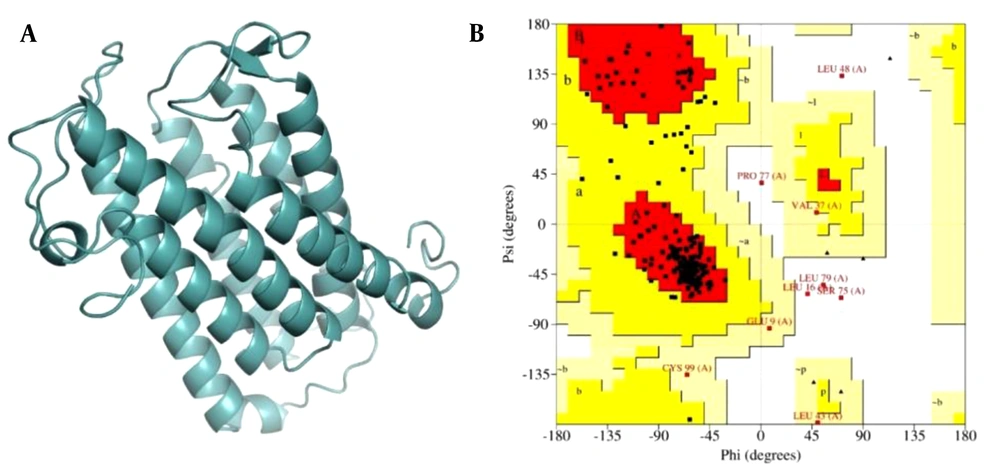
Since no three-dimensional (3D) structure of 5AR1 is currently available in the Protein Data Bank, homology modeling was utilized to generate the structure of SRD5A. Modeling approaches are the most reliable methods of predicting the 3D structure of an unknown protein based on known homologous reference protein with high identity to the novel protein as a template. The FASTA format of the 5AR1 enzyme protein sequence was retrieved from the UniProt database (UniProtKB) with the accession number P18405. Based on best sequence alignment, the 3D structure of the protein was modeled using I-TASSER online server. The model was validated using various tools, such as ERRAT (22) and PROCHECK (23), to generate the Ramachandran plot.
3.2. Dataset Collection
The data of this study contained several medicinal plants widely used in home remedy medications. The present study identified the main components of these selected herbs. Cedrol, Cedrene, and Thujopsene are detected in Cedar (Cedrus). Cedarwood essential oil is used as a skincare agent and hair growth medication (24). 1,4-naphthoquinone and Lawsone are active compounds isolated from Lawsonia inermis, also known as the henna tree. Studies evaluated that Lawsonia inermis compounds can slow hair loss and help nourish the scalp (25). Epicatechin gallate (ECG) and Epigallactocatechin gallate (EGCG) are extracted from Camellia sinensis leaves (26-28). Other compounds are β-sitosterol and stigmasterol isolated from Serenoa repens. It is a popular herbal remedy that boosts hair growth and helps maintain scalp health (29, 30). Riboflavin (vitamin B2) is a known antioxidant present in various plants. Research showed that riboflavin deficiency is associated with hair loss (31). Finasteride and dutasteride, used orally as 5AR1 inhibitors, were selected as positive controls. Figure 1 depicts the two-dimensional (2D) structure of all compounds.
3.3. Protein and Herbal Compounds Preparation
AutoDock Vina tools 1.5.6 were used to prepare 5AR1 and all compounds. The modeled structure of 5AR1 was obtained from I-TASSER online server and imported to the AutoDock Vina tools program. All water molecules were merged, polar hydrogens were added, and Kollman charge (32) and AD4 atom type were assigned. All the above-mentioned 3D structures of compounds were retrieved from PubChem data bank. Then, nonpolar hydrogens were eliminated; finally, Gasteiger charge was assigned to calculate the partial charge of all inhibitors.
3.4. Docking Studies
A molecular docking experiment was employed to determine the binding modes of selected components with 5AR1 active site residues. This study used AutoDock Vina to perform docking procedures to predict all inhibitors’ best configuration within 5AR1 protein. The grid box parameter values were adjusted with the size of 25 × 25 × 25 in specific coordinates of x, y, and z-axis (x = 64.899, y = 62.246, and z = 66.772) and the space value of 1 Å to confirm the best binding affinity of the inhibitor-protein complex. The Lamarckian genetic algorithms (33) were selected to perform docking. The best confirmation with the lowest binding energy estimated by Autodock Vina was visualized with PyMOL (34) and LigPlot plus (35) to provide a 2D diagram of the compound’s interaction against 5AR1.
4. Results
4.1. Constructed Model of 5AR1 and Validation
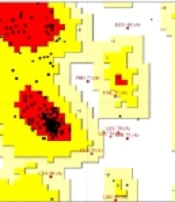
Figure 2A illustrates the constructed homology model of 5AR obtained from I-TASSER. PROCHECK and ERRAT were used to evaluate the model structure. ERRAT results showed an overall quality factor of 92.0319. The Ramachandran plot of the model in Figure 2B shows that 86.97% of residues are in the most favored regions (red), 9.8% in additional allowed regions, 1.8% in generously allowed regions, and 1.8% in disallowed regions. These statistics indicated that the modeled structure was suitable for docking simulation.
4.2. Docking Studies and Visualization
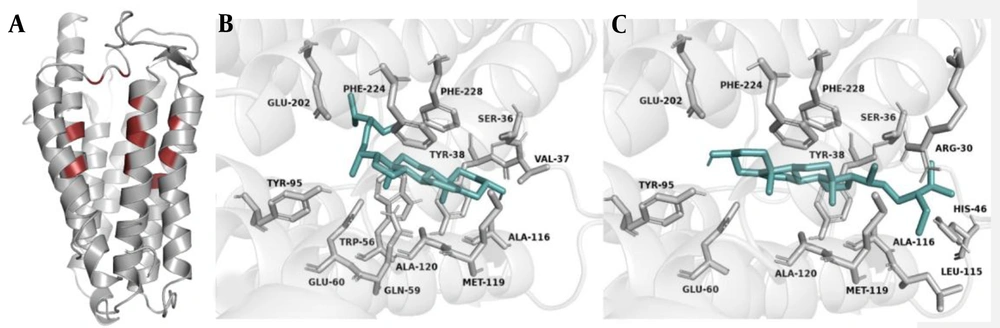
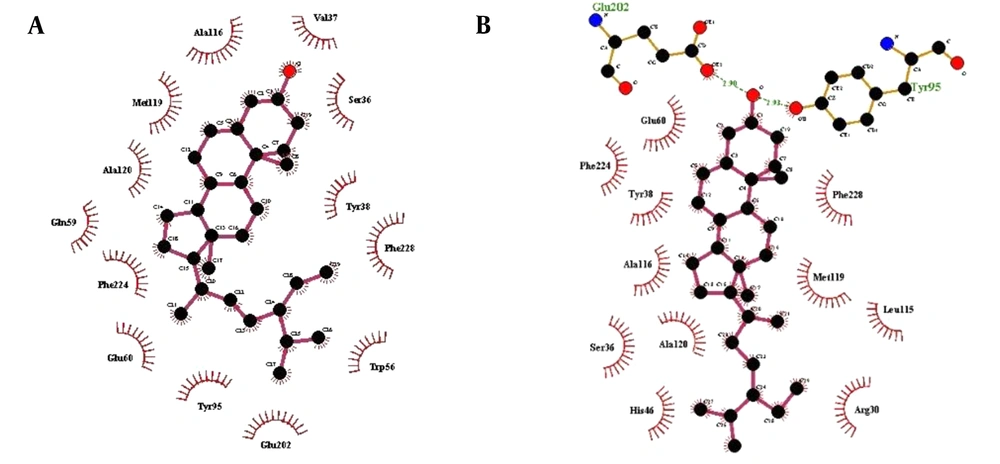
AutoDock Vina results revealed the orientation and interaction of all natural compounds against 5AR1. The most active compounds of the present study data collection are listed in Table 1. In addition, the binding energy, H bonds, and hydrophobic interactions are also shown. The docking results demonstrated that two compounds, β-sitosterol, and stigmasterol from Serenoa repens, had the strongest docking affinity of -10.7 and -10.1 kcal/mol, respectively, compared to the rest of the collection. In the second place, ECG and EGCG isolated from Camellia sinensis leaves had the binding energy values of -9.9 and -9.6. kcal/mol, respectively. Riboflavin also showed high affinity (-9.1 kcal/mol) but weaker than the last two groups and equal to or greater than finasteride and dutasteride. Cedrus’s three main compounds (ie, Cedrol, Cedrene, and Thujopsene) displayed well but were weaker than the positive controls with the binding energy of -8.2, -8.3, and -7.9 kcal/mol, respectively. Lawsone and 1,4-naphthoquinone isolated from Lawsonia inermis exhibited the weakest affinity among other compounds with the active site residues of 5AR1. Screening the interaction details of the 5AR1-inhibitor complex from LigPlot+ showed that almost every compound interacts with Ser36, Tyr38, Trp56, Glu60, Tyr95, Ala116, Met119, Ala120, Phe123, Phe224, and Phe228. It could be inferred that these residues are critical in the binding site and can be considered important residues for inhibitors to bind within the active pocket of 5AR1. The most vital interaction regions of 5AR1 are shown in brown parts in Figure 3A. The 5AR1 residues involved in the interaction with stigmasterol and β-sitosterol are represented in 3D and 2D structures in Figures 3B-3C and 4A-4B, respectively.
| Compounds | Binding Energy (kcal/mol) | H-bond | Hydrophobic Interaction |
|---|---|---|---|
| Stigmasterol | -10.7 | - | Ser36, Val37, Tyr38, Trp56, GLN59, Glu60, Tyr95, Ala116, Met119, Ala120, Glu202, Phe224, and Phe228 |
| Beta-sitosterol | -10.1 | Tyr95 and Glu202 | Arg30, Ser36, Tyr38, His46, Glu60, Leu115, Ala116, Met119, Ala120, Phe224, and Phe228 |
| Epigallactocatechin gallate | -9.9 | Tyr56, Arg176, Tyr183, Arg232, and Tyr240 | Tyr38, Gly39, Ala52, Tyr102, Met106, Gly108, Gly109, Met112, Leu172, Tyr199, and His236 |
| Epicatechin gallate | -9.4 | Arg98, Asn165, Asp169, and Asn198 | Tyr38, Trp56, Glu60, Met119, Ala120, Phe123, Glu202, Phe221, Phe224, Phe228, and Leu229 |
| Riboflavin | -9.1 | Arg98 and Glu202 | Tyr38, Trp56, Gln59, Glu60, Tyr95, Ala116, Met119, Ala120, Phe123, Phe224, Phe228, and Leu229 |
| Dutasteride | -9.1 | Arg30, Asn31, and Glu60 | Ser36, Val37, Tyr38, His46, Gln59, Trp56, Leo115, Ala116, Met119, Ala120, Phe224, and Phe228 |
| Finasteride | -8.5 | Tyr95 and Arg98 | Arg30, Ser36, Val37, Glu60, Ala116, Met119, Ala120, Phe123, Phe224, and Phe228 |
| Cedrene | -8.3 | - | Trp56, GLN59, Glu60, TYR95, Ala120, Phe123, Glu202, Phe221, Phe224, Thr225, and Phe228 |
| Cedrol | -8.2 | Glu60 | Gln59, Tyr95, Ala120, Phe123, Phe221, Phe224, and Phe228 |
| Thujopsene | -7.9 | - | Glu60, Tyr95, Met119, Ala120, Phe123, Phe221, Phe224, THR225, and Phe228 |
| Lawsone | -7.4 | Arg30 and Ser36 | Val27, Tyr38, Ala116, Met119, Ala120, Phe224, and Phe228 |
| 1,4-naphthoquinone | -7.1 | Arg30 and Ser36 | Tyr38, Ala116, Met119, Ala120, Phe224, and Phe228 |
5. Discussion
This study investigated the possible interactions between herbal inhibitors and 5AR1 protein using bioinformatics tools for AGA medication. Previously, Lin et al. worked on 5AR1 inhibitors to treat BPH; however, contrary to the present work, they used AutoDock for the docking procedure (36). In comparison to AutoDock 4, AutoDock Vina significantly increases the average accuracy of the binding mode predictions and is incredibly faster. It has always been among the six first choices for docking analysis globally. In the present study, this software could define the interactions properly.
The current study findings revealed that all inhibitors have possible interactions with 5AR1. Moreover, among the selected inhibitors, the highest binding (-10.7 kcal/mol) belongs to stigmasterol. The present analyses indicated that stigmasterol binds to 5AR1 through only hydrophobic forces, and 13 amino acid residues were detected in the interaction site (Figure 3B). Therefore, it can be concluded that stigmasterol might be the best inhibitor. This inhibitor is suggested as the new choice for further investigations in this field. In the second rank, β-sitosterol was placed, with -10.1 kcal/mol. In the interaction site of β-sitosterol, 13 amino acid residues were detected (Figure 3C). The high energy value of this inhibitor showed the possible potential of this component to inhibit 5AR1.
Similar to the present study results, previous studies confirmed the possible ability of stigmasterol and β-sitosterol to inhibit 5AR. Prager et al. established the effectiveness of β-sitosterol to control AGA. For the first time, Prager et al. used a placebo-controlled double-blind method to examine the benefit of this botanical substance (37). In addition, Cabeza et al. reported the effect of β-sitosterol to inhibit 5AR1 in the hamster prostate (38). They showed β-sitosterol could decrease the prostate weight, and this effect was not related to the binding of β-sitosterol to the androgen receptor but the inhibition of 5AR. However, both targets were present in the prostate.
Furthermore, Upadhyay et al. investigated the effect of β-sitosterol phyto-vesicles to treat AGA. The aforementioned study confirmed the ability of β-sitosterol in the control of alopecia, and phyto-vesicles could increase this compound’s water and lipid solubility (39). Finally, Chen et al. showed the potential of stigmasterol and β-sitosterol to target 5AR and inflammatory pathways, which confirmed the efficacy of these components to treat AGA and BPH. In Chen et al.’s study, in vitro assays were used. The results of the aforementioned study determined the down-regulation of messenger ribonucleic acid expression profile characteristics of both disease processes in AGA (hair follicle dermal papilla cells) and BPH (LNCaP prostate cells) cell lines treated with stigmasterol or β-sitosterol. The aforementioned study proved that stigmasterol and β-sitosterol targeting 5AR and inflammatory mediators might represent a rational approach in treating AGA and BPH (40). Currently, available drugs for AGA are finasteride and dutasteride, which are synthetic. However, these two FDA-approved drugs have good inhibitory activity. Recent studies have shown that they can lead to severe adverse effects, including ejaculation disfunction, reduction of libido, gynecomastia, and erectile disorder (41).
Docking analysis showed that dutasteride had a stronger binding affinity (-9.1 kcal/mol) than finasteride. In both inhibitors’ interaction sites, H-bonds and hydrophobic forces get involved. In line with the present study results, some previous studies showed the inhibitory activity of these inhibitors. Rabasseda et al. and Olsen et al. indicated that dutasteride increased scalp hair growth in men with MPHL and suggested that type 1 and type 2 5AR might be important in the pathogenesis and treatment of MPHL (42, 43). In addition, Gubelin Harcha et al. determined that dutasteride increased hair growth and restoration in men with AGA. Gubelin Harcha et al. showed that dutasteride 0.5 mg could remarkably increase hair width and hair terminal count more than finasteride 1 mg, suggesting that dutasteride is more effective than finasteride in hair loss restoration (44). Eun et al. cited in Dhurat and Shanshanwal showed the potential of dutasteride to inhibit 5AR (type 1 and type 2), converting testosterone to DHT (45). Moreover, Inadomi, Shapiro and Kaufman, and Yanagisawa et al. conducted different pilot studies, and their results showed that finasteride is effective in the treatment of AGA patients (46-48). Finally, Arif et al. and Jung et al. introduced dutasteride as a treatment of choice for AGA (49, 50).
Liao et al. showed that EGCG and ECG extracted from green tea could inhibit 5AR in the sex gland in the rat. Furthermore, Hiipakka et al. determined that EGCG can affect 5AR in cell-free but not whole-cell assays. They suggested that EGCG with long-chain fatty acids is active in both cell-free and whole-cell assay systems (26, 51). Koseki et al. suggested the potential of ECG to inhibit 5AR, which results in inhibiting the androgen-related pathogenesis of acne, testosterone conversion, and sebum synthesis. Therefore, it was proposed that it can be a helpful agent in the therapeutic strategy of acne (52). The present study confirmed the possible interactions between EGCG and ECG with 5AR by computational methods, similar to the aforementioned studies. Both inhibitors’ energy values were about -9 kcal/mol.
The docking results of the present study estimated the strong interaction with -9 kcal/mol binding energy. Moreover, Nakayama et al. introduced riboflavin as a 5AR inhibitor. It was confirmed by Cho et al. that riboflavin has stronger potent inhibitory activity than other compounds, such as emodin and alizarin (53, 54). Although the binding energy of Cedrol (-8 kcal/mol) is not close to the highest ones, it was predicted to be a strong inhibitor. Zhang et al. showed the beneficial effect of Cedrol on hair loss and confirmed that it has a strong hair growth promotion effect (55). In addition, Deng et al. suggested that Cedrol nanoemulsion significantly improved pharmacokinetic properties and hair growth (56).
1,4-naphthoquinone was extracted from a natural source for the first time by Ishiguro et al. that showed the significant testosterone 5AR inhibitory activity (57). Although Ishiguro et al. (57) examined the inhibitory activity of 1,4-naphthoquinone, the current study findings showed that it has the lowest binding energy (-7.1 kcal/mol), compared to other inhibitors. Therefore, it can be concluded that this inhibitor has the weakest interaction with 5AR1. Although Lawsone, Thujopsene, and Cedrene were listed as 5AR1 inhibitors in some reviews, any experimental studies related to these components were detected. By considering the docking results of the present study, it can be concluded that among these inhibitors, Cedrene is more capable of inhibiting 5AR1 due to the highest binding affinity (-8.3 kcal/mol), compared to Thujopsene and Lawsone with binding energy values of -7.9 and -7.4 kcal/mol, respectively.
Docking analysis showed that some regions were critical in the interaction between selected inhibitors and 5AR1, shown in Figure 3A as brown regions. Among amino acid residues involved in all interactions, 11 amino acids (ie, Ser36, Tyr38, Trp56, Glu60, Tyr95, Ala116, Met119, Ala120, Phe123, Phe224, and Phe228) were detected in the majority of inhibitors and 5AR1 interaction sites. Therefore, it can be suggested that the aforementioned residues are essential to inhibit this protein and might be helpful in designing new targets for the next generation of 5AR1 inhibitors.
5.1. Conclusions
The present study aimed to examine the potential activity of herbal components as 5AR1 inhibitors using in-silico analysis, which can be helpful for AGA treatments and restoration of hair follicles. In conclusion, the present predictions determined two inhibitors (ie, β-sitosterol and stigmasterol) with the most robust interactions as the suggested compounds to be used in further investigations as the next generation of drugs to control AGA.