1. Context
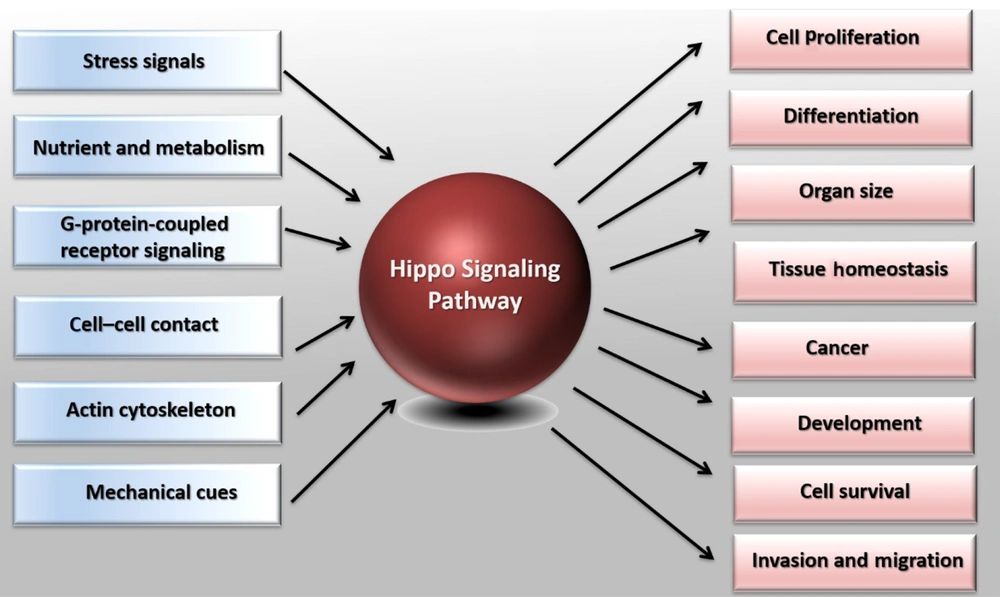
Regulation of the cell number plays a vital role in tissue growth and homeostasis, and its dysregulation leads to tumorigenesis or organ degradation (1, 2). It has been shown that the Hippo signaling pathway (also known as Salvador-Warts-Hippo) plays a vital role in these processes (3). In Drosophila and vertebrates, the cell number modulates cell proliferation processes, cell death, and cell differentiation (4). During growth and development, cell proliferation is necessary for expanding the size of the organs and the body. In addition, the proper cell differentiation ensures the proper function of organ growth. Aged or damaged cells go through cell death, and adult stem cells divide and differentiate to replace them (5, 6). Under pathological conditions such as injuries and organ repair, cell division and differentiation of tissue-specific progenitor cells are increased to compensate for lost cells (7). On the other hand, uncontrolled cell proliferation and reduced cell death lead to tumorigenesis (8-10). The detailed mechanisms associated with cell proliferation, cell death, and cell differentiation have been widely studied. However, it is not yet completely understood how these processes are regulated (Figure 1).
Demonstration of the Hippo pathway in cell biology. The Hippo pathway responds to diverse stimuli, such as G-protein-coupled receptor signaling, nutrient, and metabolism, cell-cell or cell-matrix contact, mechanic cues, actin cytoskeleton, and various stress signals. Based on the stimuli, the Hippo pathway controls different biological processes, including cell proliferation, organ size, and cell survival. The Hippo pathway also plays a crucial role in tumorigenesis, differentiation, organ development, and homeostasis.
The Hippo pathway modulates cell proliferation, differentiation, and death processes. The Hippo pathway's regulation of many cellular processes indicates the essential role of this pathway in various physiological and pathological conditions, such as growth, evolution, tissue homeostasis, and tumorigenesis (5). The Hippo pathway has a unique capacity for tumor formation and progression. Mutations and dysregulated expression of its main components (MST1/2, LATS1/2, YAP, TAZ) can cause cancer cells' migration, invasion, and malignancy. The biological importance of this pathway in tumorigenesis has received much attention in the last few years. Therefore, in this review study, we examine the function of different components of the Hippo signaling pathway, its molecular regulation, and its involvement in tumorigenesis and cancer progression. Finally, we will investigate the role of this pathway in cancer treatment.
2. Evidence Acquisition
2.1. Hippo Signaling Pathway
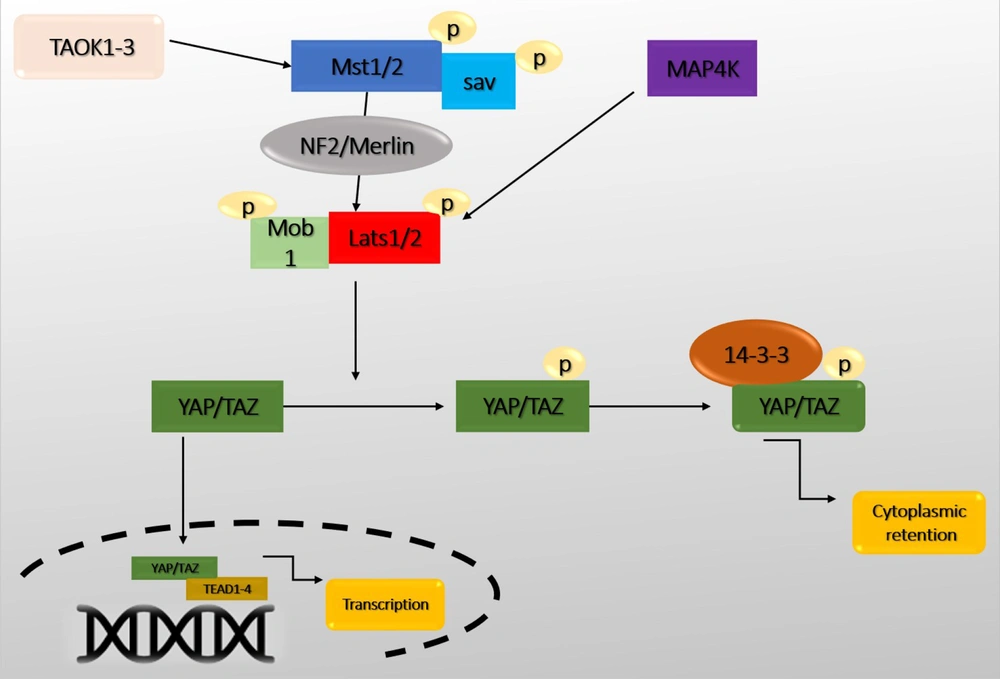
Recently, the Hippo pathway has been identified as a pathway that induces cell differentiation and death and prevents cell proliferation. Therefore, the Hippo pathway can be introduced as a critical pathway in coordinating these biological processes (11). In addition, this pathway has a role in tissue growth control, cell fate determination, mitosis, and pluripotency (12). Dysfunction of the Hippo pathway has also been reported in many human cancers (13). The Hippo pathway was first identified in Drosophila and defined by demonstrating genetic mosaicity for tumor suppressor genes (14). Genetic inactivation of genes, including Warts (Wts), Hippo (Hpo), Salvador (Sav), and Mats, can develop similar phenotypes with increased tissue growth. Yorkie (Yki) is one of the critical downstream targets in the Hippo pathway that regulates transcription by interacting with the Scalloped (Sd) transcription factor (15). The Hippo pathway is highly conserved among mammals. Alteration of its activity leads to significant changes in the size of organs, especially the liver (16). Macrophage stimulating 1/2 (Mst1/2) (ortholog Hpo), large tumor suppressor 1/2 (Lats1/2) (ortholog Wts), Sav1, and MPSON binder 1 (Mob1) (ortholog Mobkl1A, Mobkl1B, Mobkl1C) construct a kinase cascade that phosphorylates and inhibits Yes-associated protein 1/Tafazzin (YAP/TAZ) (ortholog Yki) upon activation. YAP/TAZ performs essential physiological functions in the Hippo pathway through binding to transcriptional enhancer factor (TEF) and ABAA domain (Tead 1-4) (Sd orthologs) (Figure 2). The core members of the Hippo-pathway are well-identified in Drosophila and mammals, but this pathway's regulatory mechanisms have been rarely studied (17, 18).
The central core of the Hippo pathway. Macrophage stimulating 1/2 (Mst1/2) leads to the phosphorylation of Sav, large tumor suppressor 1/2 (Lats1/2), MPSON binder 1 (Mob1), and Lats1/2 phosphorylate Yes-associated protein 1/Tafazzin (YAP/TAZ). Subsequently, phosphorylated YAP/TAZ interacts with 14-3-3, resulting in the retention and degradation of YAP/TAZ in the cytoplasm. Without nuclear Yap, the expression of transcriptional enhancer factor (TEF) and ABAA domain (Tead) target genes is suppressed. In contrast, when YAP/TAZ is dephosphorylated (the Hippo signaling is inactive), they enter the nucleus and control the expression of a group of genes by interacting with Tead1-4 and activating the transcription of target genes.
Mst1/2 are protein kinases of the sterile 20 (STE20) family that phosphorylate Sav, Lats1/2, and Mob1. Mst1/2 kinetic activity is enhanced by interacting with Sav, mediated by SARAH domains in Mst1/2 and Sav (19). In addition, thousand and one (TAO) or TAOK1-3 kinase has been shown to phosphorylate and activate Mst1/2 or Hpo directly (20). In Drosophila, Ras-assocaition family (RASSF) competes with Sav and leads to dephosphorylation and inactivation of Mst1/2 by adsorption of the protein phosphatase 2A (PP2A) complex. Mst1/2 directly phosphorylates and activates Lats1/2. Sav1 acts as a bridge to bring Mst1/2 and Lats1/2 together (21). There is also some evidence that MST1/2 are activated without upstream kinases. They are only activated by autophosphorylation of MST1/2. Another critical component called NF2/Merlin facilitates Lats1/2 phosphorylation by the MST1/2-Sav complex. When Mst1/2 is active, there are two groups of MAP4Ks called MAP4K1/2/3/5 (Hppy ortholog) and MAP4K4/6/7 (Msn ortholog) that directly phosphorylate LATS1/2 (6, 22).
Lats1/2 phosphorylates YAP/TAZ directly. When YAP/TAZ is phosphorylated, it remains in the cytoplasm due to its interaction with the protein 14-3-3. Therefore, the transcription of its target genes is inhibited. Whereas, when upstream kinases are inactivated, dephosphorylated YAP/TAZ enter the nucleus and regulate gene expression. YAP/TAZ phosphorylation also regulates their protein stability (23). Recently, several proteins have been discovered that directly regulate YAP displacement and activation without Lats (5). YAP/TAZ does not have a DNA-binding domain, so they bind to the promoter of target genes by interacting with DNA-binding transcription factors. YAP/TAZ primarily binds to TEAD1-4 transcription factors and control genes involved in cell proliferation and death (5). TEAD1-4 can bind to VGLL4 in the nucleus and act as a transcriptional suppressor. The interaction between YAP/TAZ and TEAD1-4 causes the separation of VGLL4. Also, TEAD-mediated activation induces tissue growth and inhibits apoptosis (24). In addition to TEADs, YAP/TAZ interacts with other transcription factors, such as Smad1, Smad2/3, Smad7, RUNX1/2, P63/P73, and ErbB4. It also controls the transcription of various genes involved in proliferation, differentiation, and growth (25) (Table 1).
| Drosophila | Mammals | Main Function |
|---|---|---|
| Hippo (Hpo) | Mst1/2 | Phosphorylation of Lats1/2 and Mob1 and Sav1 |
| Salvador (Sav) | Sav1 | Interaction with Mst1/2 and promotion of Lats phosphorylation |
| Warts (Wts) | Lats1/2 | Phosphorylation and inactivation of YAP/TAZ |
| Mats | Mob1 A/B | Scaffold protein LATS1/2 |
| Yorkie (Yki) | YAP/TAZ | Helper for transcription activator (main pathway elements) |
| Scalloped (Sd) | TEAD1-4 | Transcription factor |
| Tgi | VGLL4 | YAP/TAZ function inhibitor |
| Merlin | Merlin/NF2 | Facilitate Lats activation by Mst1/2 |
2.2. Regulation of the Hippo Pathway in Physiological and Pathological Conditions
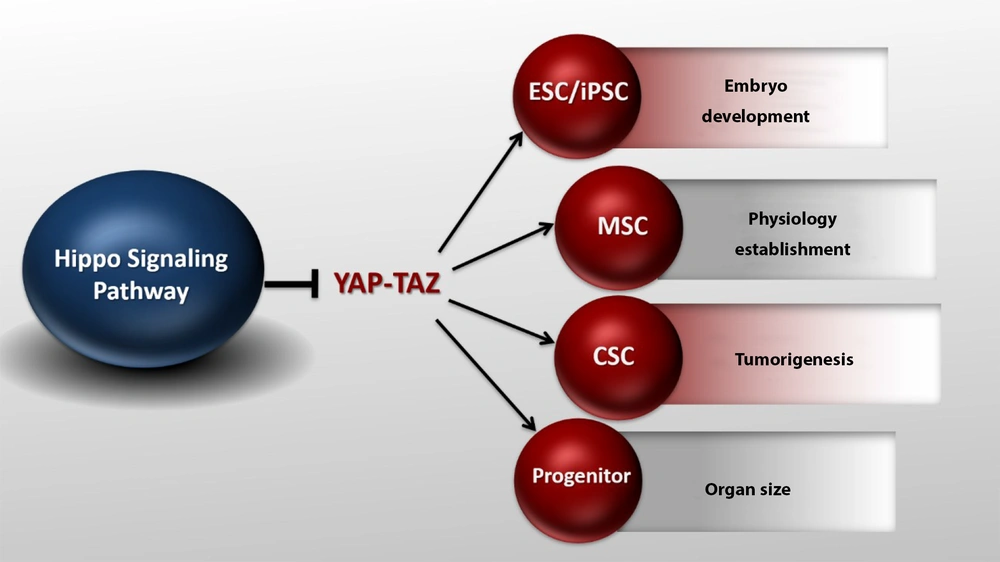
In recent years, the Hippo pathway has been shown to regulate stem cell function. YAP/TAZ is required to maintain pluripotency in mouse and human embryonic stem cells (26). Increased YAP/TAZ activity in transgenic mice promotes tissue-specific stem cell proliferation and blocks cell differentiation (17). YAP/TAZ is also necessary for the differentiation of mesenchymal stem cells. Silencing YAP/TAZ in these cells reduces osteogenesis and increases lipid production (27). In addition, many studies have suggested the role of YAP/TAZ in the differentiation of muscle cells (28). These results indicate that the Hippo pathway plays an essential role in cell differentiation. The Hippo pathway is also involved in tissue repair (29). In the mouse intestine, the amount of YAP protein is significantly increased due to injuries, which promotes the regeneration and repair of the epithelium of the damaged intestine. However, YAP inactivation intensely leads to incomplete repair and reconstruction (30). YAP plays a vital role in early embryonic development. In mice, YAP silencing is lethal as it stops fetal growth on day 8.5. This leads to abnormalities in the yolk sac, angiogenesis, and elongation of the body axis (31). The Hippo pathway is known as the signaling pathway controlling the organ size. In Drosophila, the loss of function mutations in Hpo, Sav, Wts, and Mob results in significant tissue growth, as shown by large eyes and wings (5). Increased Yki expression also shows a similar increase in tissue growth (32). In mammals, the Hippo pathway function is protected to control the organ size. For example, increased expression of YAP, specifically in rat liver and heart tissue, results in a significant increase in both organs.
In addition, the silencing of Mst1/2 and Sav in the liver and heart leads to an increase in size (33). Parts of this pathway have been identified as tumor suppressors in Drosophila. The kinases present in the pathway are usually tumor suppressors. On the other hand, YAP/TAZ has a proto-oncogenic function (34). Increased expression of YAP/TAZ has been widely found in human cancers (35). Transgenic mice overexpressing YAP also obtain tumors and hyperplasia (36). Similarly, inactivation of major and upstream members of the Hippo pathway leads to tumor metastasis in mice (37, 38). In addition, NF2 inactivation, a tumor suppressor that acts upstream of the Hippo pathway, leads to increased tissue growth in Drosophila and cancer in mice (39, 40) (Figure 3).
Yes-associated protein 1/Tafazzin (YAP/TAZ) activity in different conditions. YAP/TAZ activity can affect stem cells and embryo development, organ size, and tumorigenesis. The Hippo pathway can modulate its effects on tissue size, cancer, and organ development by directly regulating stem cells (ESC, MSC, CSC, progenitors) proliferation and maintenance.
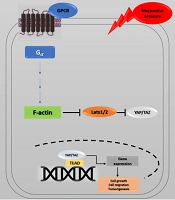
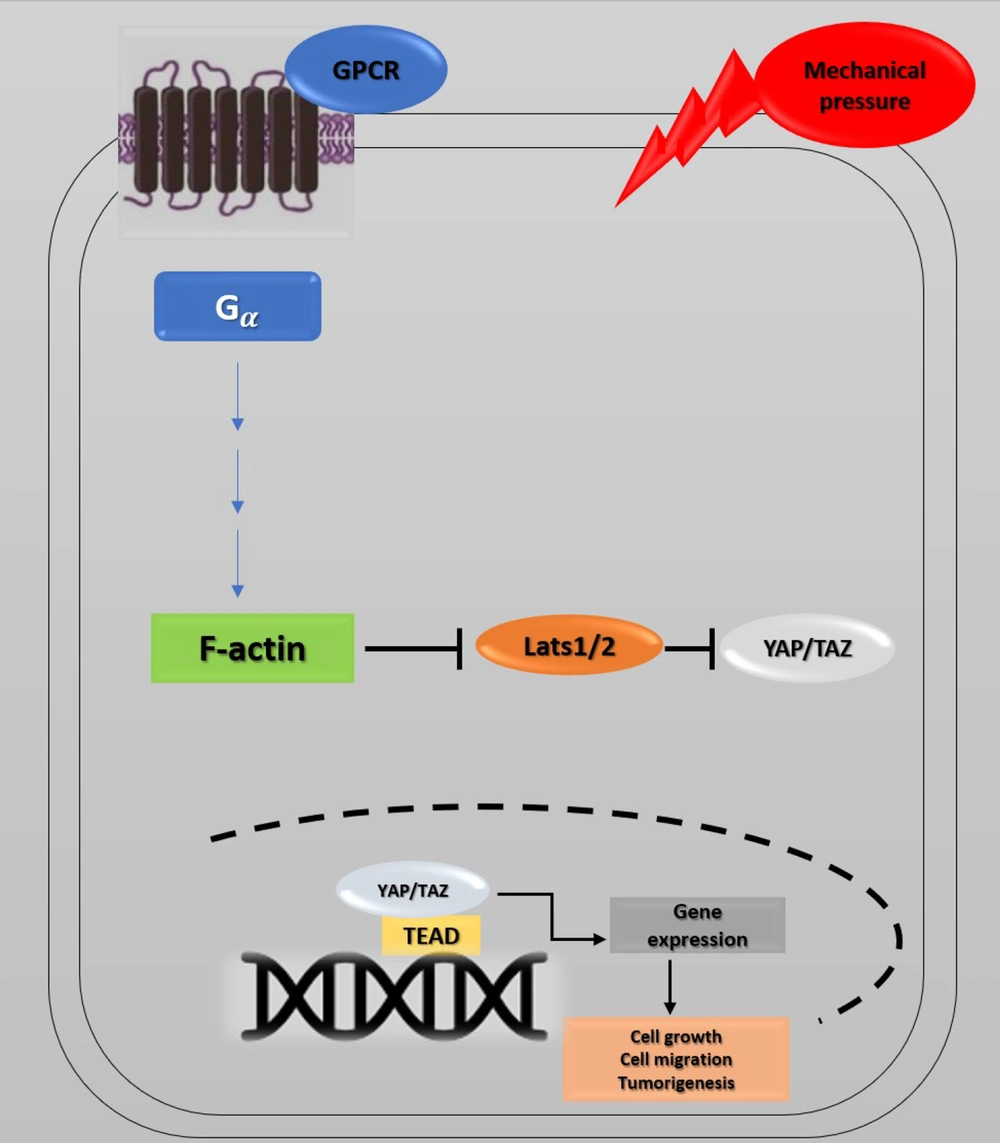
Recent studies have investigated YAP's role in the progression of cancer metastasis in mice and TAZ in maintaining the self-renewal of breast cancer stem cells (41). This information indicates the critical role of the Hippo pathway in cancer metastasis (42, 43). The correlation between the Hippo pathway, mechanical stress, and the G protein-coupled receptor (GPCR) signaling pathway has also provided a new perspective on cancer metastasis. GPCRs are cell membrane proteins that attach to their ligands outside the cell and transmit signals from these materials to an intracellular molecule called G protein. Cells may form wider local adhesions to the extracellular matrix following the loss of cell-cell junctions and loss of cellular polarity. As a result, they are more likely to stimulate GPCR ligands (5, 44). Under such conditions, YAP/TAZ activation facilitates cell proliferation and migration. GPCRs are vital factors in cancer promotion whose dysregulation has been identified in various human cancers. GPCR signaling has several roles in cancer development (45) (Figure 4). Overall, the activation of YAP/TAZ through dysregulated GPCR signaling plays an essential role in promoting cancers (46).
Hippo pathway and GPCR signaling. Yes-associated protein 1/Tafazzin (YAP/TAZ) activity is controlled by cell density, extracellular matrix, and GPCR signaling. Elimination of cell junctions induces mechanical stress, leading to changes in cell morphology and YAP/TAZ localization in the nucleus. Through the activation of G proteins and possibly rearrangements of the actin cytoskeleton, G protein-coupled receptor (GPCR) signaling can potently modulate the phosphorylation states and activity of YAP/TAZ. Ectopic GPCR signaling can activate YAP/TAZ. High expression of GPCR or Gα-activating mutations may cause YAP/TAZ nuclear localization, leading to cancer proliferation.
2.3. Hippo Signaling Pathway in Cancer
The Hippo signaling pathway regulates many tumorigenic processes (47). The Hippo pathway regulates many cellular features associated with tumorigenesis. This pathway is regulated by cellular properties such as cellular polarity, cell-cell adhesion, contact inhibition process, and mechanical cues. On the other hand, it controls cell proliferation, cell survival, maintaining stem cell characteristics, metastasis, cell recovery, and regeneration (42). Disorders in the Hippo pathway have been reported frequently in many human carcinomas, including lung, colorectal, ovarian, liver, and prostate cancers (48). Some Hippo pathway members have been identified as tumor suppressor genes reduced in cancer (49). Other parts have been reported as oncogenes increasing in cancer (30, 50, 51). Also, the core members of this pathway, including YAP/TAZ activators and Tead1-4 transcription factors, have been reported as oncogenes. Two general mechanisms are involved in the dysregulation of the Hippo pathway, including changes in the expression and mutations of pathway members and Hippo pathological disorders associated with other signaling pathways (20, 52). Today, this pathway is also considered a therapeutic target in cancer (53). Since the Hippo kinase pathways and YAP are key pathway activators, they can apply for primary therapeutic purposes. Usually, kinases are considered the best targets for treatment with macromolecules (54).
2.4. Hippo Pathway and Cancer Treatment
Any changes in the YAP/TAZ level in the nucleus can cause a variation in the transcription of the target genes (35). High levels of dephosphorylated YAP/TAZ proteins are present in active and dividing cells or cancer cells, increasing the cell's primary activities, including cell proliferation and tumorigenesis (55). According to the proposed classical model, the YAP/TAZ cytoplasmic-nuclear exchanges are not binary but react to a continuous spectrum of cytoplasmic-nuclear currents (35). In the nucleus, excessive YAP/TAZ with increased TEAD expression increases drug resistance in most cancers, including breast, gastric, urinary, genital tract, female reproductive organs, skin, bone, and brain cancers (56, 57).
On the other hand, disruption and especially the lack of upstream tumor inhibitors in the Hippo pathway activate YAP in the nucleus, which, in turn, activates tumorigenic promoters (58). Therefore, YAP/TAZ nuclear localization inhibition can be used as a therapeutic method (35). Drugs that indirectly prevent YAP/TAZ from accumulating in the nucleus can suppress the transcriptional signaling pathway activating the target genes (59). For example, Dasatinib, Statins, and Pazopanib are drugs that block the YAP/TAZ nuclear localization in breast cancer cells (60). There are key potentially therapeutic sites in protein-protein interactions between YAP/TAZ and TEAD (55).
The wound-healing process in injured tissue is accompanied by the release of cytokines and transcription factors that activate and differentiate fibroblasts from myofibroblasts. Myofibroblasts are responsible for fibrogenesis and the production of Extracellular Matrix (ECM) components such as collagen, laminin, and fibronectin (61). In the case of chronic injury associated with prolonged inflammation, myofibroblasts' intensity increases ECM production, resulting in fibrosis. Also, myoblasts are the primary keys to promoting fibrosis, metastasis, invasion, and tumor formation under stressful conditions. It has recently been shown that the Hippo pathway is involved in fibrosis and tumorigenesis due to ECM changes by myofibroblasts (62). Recent findings indicate that YAP/TAZ and ECM work together to increase matrix strength, thus increasing YAP/TAZ oncogenic activity (63). YAP/TAZ induces cell differentiation of fibroblasts into myofibroblasts. They can also cause fibrosis in almost all vital organs such as the lungs, kidneys, liver, heart, and skin by different mechanisms. The activation of YAP/TAZ in response to matrix stiffness also causes tumorigenesis. Increased ECM stiffness increases YAP cellular activity, leading to the expression of its target genes. Therefore, inhibitory drugs that target the Hippo pathway can help treat both fibrosis and cancer. In the following, we will review the articles published in recent years and introduce and describe the mechanisms of drugs that target different components of the Hippo pathway, especially YAP/TAZ (64).
2.5. Hippo Pathway as Therapeutic Targets
Decursin is a compound isolated from the Dang-Gui plant's roots, which has recently been shown to have anti-cancer therapeutic uses. Decursin enables the inhibition of YAP activity in liver cancer cells (65). Cucurbitacins are tetracyclic compounds derived from several plant families, such as Cruciferae and Cucurbitaceae. These compounds have various medicinal activities, including antioxidant, anti-cancer, and anti-diabetic functions. Chai et al. reported the anti-cancer activity of Cucurbitacin B in colorectal cancer cells by inhibiting YAP protein (66).
Verteporfin is a small molecule that inhibits the interaction between YAP and TEAD, thereby suppressing YAP function (67, 68). Several researchers have found that Verteporfin can hinder the growth of cancer cells in some tumors, such as retinoblastoma and ovarian cancer. In addition to Verteporfin, other members of the porphyrin family, such as hematoporphyrin and protoporphyrin IX, are currently known to disrupt the YAP-TEAD interaction in mouse models. These can be considered the future candidates for cancer treatment (69). Dimethyl fumarate also significantly inhibits the nuclear localization of YAP in skin fibroblasts of patients with systemic sclerosis, thus preventing the development of dermal fibrosis (55, 70). According to recent studies, due to the relationship between the cytoskeleton and the intracellular localization of YAP/TAZ, cytoskeletal-modifying drugs can be used to target YAP and TAZ (71). For example, the inhibition of Rho-kinase has been shown to reduce the localization of YAP/TAZ, thereby reducing tumorigenesis (72). In addition, experiments have shown that the members of the Hippo pathway can be involved in drug resistance. For example, increased YAP/TAZ expression and dephosphorylation induce taxol resistance in several cancer types (58, 73).
About two decades ago, the Hippo pathway was identified when studying tissue growth in Drosophila. Then, it was widely studied in mammals. The regulation of the Hippo pathway is essential, as it controls tissue growth, mitosis, cell pluripotency, cell proliferation, differentiation, survival, growth, migration, death, and organ size. The core members of the Hippo pathway control the expression of a group of genes by phosphorylating and dephosphorylating YAP/TAZ. The Hippo pathway can affect cell carcinogenesis through different mechanisms. Also, these factors can make cancer cells resistant to existing therapies. Therefore, researchers are trying to find new therapeutic drugs targeting the Hippo pathway directly or indirectly. Computer modeling identified a unique compound called CPD3.1, which disrupted YAP interaction with TEADs. It also suppressed TEAD transcriptional activity, cell proliferation, and cell migration (74).
Similarly, MYF-01-37 has recently been identified to disrupt TEADs-YAP/TAZ binding by covalent binding to TEADs. According to studies, treatment of patients with MYF-01-37, combined with EGFR and MEK inhibitors, will target EGFR-mutated lung cancer cells (75).
Vestigial-like protein family members (VGLL1 – VGLL4) factors interact with TEADs through their Tondu domains (TDUs) and are considered TEAD transcription activators. VGLL4 is also known to be a tumor suppressor that reduces the formation of gastric cancer. Mechanically, VGLL4 competes with YAP to bind to TEADs via the TDU domain and thus acts as a YAP antagonist. However, the mechanical details of how VGLL4 is regulated in cancer are unclear (76). Despite much research about the Hippo signaling pathway, there are still ambiguities in choosing the best, most effective, and safest treatment approaches. Therefore, further studies are needed to discover mechanisms or molecules that regulate YAP/TAZ activity to provide promising therapeutic purposes in cancer progression.