1. Background
Diabetes is a metabolic disease characterized by elevated blood sugar which requires proper control (1). Type 2 diabetes is characterized by insulin resistance and decreased insulin production (2). Studies have shown that by 2049, about 629 million people will be affected diabetes (3). Type 2 diabetes is one of the main factors that elevate the risk of macrovascular and microvascular diseases and subsequent mortality (4). Some studies have shown that serum resistin level is associated with obesity and insulin resistance in people with type 2 diabetes (5, 6). Hypothyroidism is a common complication caused by insufficient production of thyroid hormones (6), which reduces metabolic and physiological activities. Its presentation include changes in weight and sleep patterns, decreased metabolic rate, decreased epithelial cell growth and division, and delayed wound healing (7). Thus, when the body needs higher levels of metabolic activity, hypothyroidism poses problems (8). Thyroid disorders are accompanied by extensive changes in metabolism, including weight, insulin resistance, and lipid profile (9). One study found that patients with Graves’ disease have decreased levels of serum resistin, while in patients with Hashimoto’s disease and goiter, it increases (10).
Several studies have examined the association between serum resistin levels and type 2 diabetes or hypothyroidism. Santili and colleagues investigated the association between circulating resistin levels and insulin resistance, oxidative stress, and platelet activation in patients with type 2 diabetes and reported that resistin reduced the insulin function (11). Kapłon‐Cieślicka and colleagues examined resistin as a prognostic factor in diabetes and showed that higher resistin levels were associated with increased risk of mortality in patients with type 2 diabetes (12). Hedayati and colleagues examined serum resistin levels in patients with hypothyroidism and hyperthyroidism and showed that patients with thyroid dysfunction have increased serum resistin (13). Verbovaia and colleagues studied the levels of resistin and other adiponectins in patients with hypothyroidism and reported that increased insulin resistance in women with hypothyroidism results in compensatory hyperinsulinemia, and people with hypothyroidism had increased concentrations of leptin and resistin (14). Literature review revealed that, to date, no study has investigated the cumulative effect of diabetes and hypothyroidism on serum resistin levels.
2. Objectives
Thus, the present study intended to investigate the cumulative effect of diabetes and hypothyroidism comorbidity on serum resistin levels in patients visiting Imam Reza Military Hospital.
3. Methods
This case-control study was conducted at Imam Reza Hospital (affiliated to the Army of the Islamic Republic of Iran) during 2018 - 2019. In total 121 patients were selected using the convenience sampling method among patients who were visiting the hospital to receive diabetes or hypothyroidism-related services. The inclusion criterion was being diagnosed with diabetes, hypothyroidism, or both. Participants were divided into 4 groups of 30 people with diabetes, 32 people with hypothyroidism, 30 people with diabetes + hypothyroidism, and 29 healthy individuals. It should be noted that participants with hypothyroidism were on thyroid medication at the time of the study and were at the euthyroid state. A blood sample was taken from all participants, who all were on fasting for 8 to 10 hours. Before blood sampling, participants rested in a sitting position for a few minutes, and then, 10 cc of blood was taken from the cubital vein. The blood samples were left at room temperature for 20 minutes to be clotted and then tubes containing the samples were centrifuged at 3000 rpm for 20 minutes. The serum was separated and stored at -80°C. A commercial ELISA kit (Shanghai Crystal day Biotech Company, Shanghai, China) was used to measure resistin levels. Levels of thyroid-stimulating hormone (TSH) were transcribed from recent lab reports of the participants. Data were analyzed using SPSS version 23. Pearson’s correlation test, ANOVA, and Tukey’s post hoc test were used to analyze the data. A P-value of < 0.05 was used as statistically significant. Patients did not pay any expenses (e.g., for transportation or laboratory tests), and the research team paid all the costs. Participants gave written and oral informed consent to all stages of the study and were free to leave the study whenever wanted. The research did not interfere with the process of treatment of the participants.
4. Results
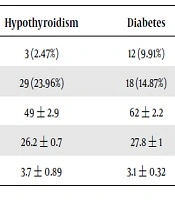
34 (28.1%) participants were male, and 87 (71.9%) were female. The youngest and oldest participants were 21 and 84 years old, respectively; the mean age of participants was 55.46 ± 14.58 years. The mean BMI of the participants was 27.1 ± 0.8. Other demographic information are presented in Table 1.
| Demoghraphic Information | Diabetes + Hypothyroidism | Hypothyroidism | Diabetes | Healthy |
|---|---|---|---|---|
| Male | 4 (3.30%) | 3 (2.47%) | 12 (9.91%) | 15 (12.39%) |
| Female | 26 (21.48%) | 29 (23.96%) | 18 (14.87%) | 14 (11.57%) |
| Age | 55 ± 1.3 | 49 ± 2.9 | 62 ± 2.2 | 43 ± 1.7 |
| BMI | 28.9 ± 0.9 | 26.2 ± 0.7 | 27.8 ± 1 | 25.7 ± 0.5 |
| TSH | 3.7 ± 0.4 | 3.7 ± 0.89 | 3.1 ± 0.32 | 3.4 ± 0.32 |
aThe data are expressed as the number of people (percent) and mean ± standard deviation.
No significant correlation was found between the serum resistin and TSH levels in all subjects (P = 0.94, r = 0.104). However, resistin and TSH levels had a moderate correlation in patients with hypothyroidism (P = 0.001, r = 0.580). Furthermore, no significant correlation was observed between resistin levels and BMI in all subjects (P = 0.532, r = 0.058).
According to the results of the ANOVA, there was a statistically significant difference in resistin levels among the study groups (P = 0.000, F = 6.813). Tukey’s post hoc test showed that the serum resistin level in patients with hypothyroidism or diabetes was significantly higher than healthy individuals and patients with both diabetes and hypothyroidism. There was no significant difference between serum resistin levels of healthy individuals and patients with diabetes + hypothyroidism. Similarly, no significant difference was detected between the serum resistin levels of patients with diabetes and patients with hypothyroidism (Table 2).
| Diabetes + Hypothyroidism | Hypothyroidism | Diabetes | Healthy | F | P | |
|---|---|---|---|---|---|---|
| Resistin | 34.3 ± 3.9A, B | 1090.1 ± 200.9C | 607.4 ± 100C | 100.9 ± 12.1 | 6.813 | 0.000 |
aData were analyzed by ANOVA and Tukey’s post hoc test and expressed as mean ± standard deviation.
bThe A, B and C symbols denote significant differences with the healthy, diabetic, and hypothyroid groups, respectively.
A significant difference was found among study groups concerning the BMI (P = 0.021, F = 3.37). There was no significant difference between healthy individuals and patients with diabetes or hypothyroidism in terms of BMI. The BMI of patients with both diabetes and hypothyroidism was higher than that of the healthy individuals, but it was not significantly different from that of the diabetic patients and patients with hypothyroidism (Table 3).
| Diabetes + Hypothyroidism | Hypothyroidism | Diabetes | Healthy | F | P | |
|---|---|---|---|---|---|---|
| BMI | 28.9 ± 0.9a | 26.2 ± 0.7 | 27.8 ± 1.0 | 25.7 ± 0.5 | 3.37 | 0.021 |
aIndicates significant differences with the healthy group.
5. Discussion
Type 2 diabetes leads to a situation in which several metabolic and hormonal disturbances collectively affect serum resistin levels. Studies have shown an increase in resistin levels in patients with type 2 diabetes (15). Thyroid hormones regulate energy levels in the body and influence the body’s adipokine levels (16) leading to a decrease in serum resistin levels in patients with Graves’ disease and an increase in patients with Hashimoto’s or goiter (10). Studies show that numerous factors affect resistin function, including the status of nutrition, antioxidants, physical fitness, and physical activity (17). Resistin was initially studied for its relationship with type 2 diabetes and obesity, but recent studies show that it is involved in inflammation and inflammatory diseases such as atherosclerosis and arthritis as well (18-20). A strong association has been observed between increased insulin resistance and increased resistin levels in obese individuals. However, resistin is also produced in inflammatory conditions and in macrophages (21-23).
According to the results, serum resistin level was significantly different among the study groups, and it was significantly higher among diabetic patients and patients with hypothyroidism than healthy individuals, which is consistent with the results of Hedayati and colleagues (13) and Kapłon‐Cieślicka and colleagues (12). There was no significant correlation between resistin and thyroid-stimulating hormone levels, but there was a moderate correlation between serum resistin and thyroid-stimulating hormone level in patients with hypothyroidism. This finding is in agreement with the results obtained by Hedayati and colleagues (13) and the study of Akbaba and colleagues (24), while it is inconsistent with the results obtained in the study of Eke Koyuncu and colleagues (25).
Overall, there was a significant difference concerning the BMI of participants in various groups, but BMI was not significantly different between healthy people and patients with diabetes or patients with hypothyroidism. In which can be attributed to the fact that the BMI of most of the participants was in the lower range and that in humans, resistin is secreted at very low levels in adipocytes, while it is also produced in mononuclear leukocyte and macrophage immune cells, and in the spinal cord and bone marrow cells. Also, low levels of resistin are produced in the lung and placental tissue (26). The findings of the present study are consistent with the results obtained by Hedayati and colleagues (13) who investigated the serum resistin in patients with hypothyroidism, but these findings are not in agreement the results obtained by Azab and colleagues (27) and Cebeci and colleagues (28) who investigated resistin in people with diabetes.
Based on the results, the serum resistin levels in patients with both diabetes and hypothyroidism were significantly lower than in those with only one of these diseases. Thus, a cumulative effect for diabetes and hypothyroidism on resistin levels cannot be inferred from the results of the present study. Further studies with larger scales and comparing the results with those obtained in the present study are recommended.