1. Background
Oxidative stress occurs as a result of imbalance between the production of oxidative factors and antioxidant defenses in cells (1). Free radicals, as the most discussable oxidative agents, have drawn lots of attention in scientific researches. They can destroy macromolecules such as lipids, proteins, and nucleic acids and cause disorders leading to numerous diseases (2). Research shows that oxidative stress plays a significant role in ischemic-reperfusion injury (3). Fatty acids (FAs) play important structural and functional roles in the cardiac cells. In addition, lipids are known as the regulatory molecules which participate in cell signaling. Moreover, they are considered to be one of the cell apoptosis agents in response to oxidative stress (4).
Lipotoxicity is a phenomenon known as the rebellious outcome of the accumulation of extra lipids in non-adipose tissues, such as cardiac muscle cells (5). Since cardiomyocytes have a limited capacity for de novo fatty acid synthesis, they depend on uptaking free fatty acid (FFA) from bloodstream. FFAs attached to albumin can be absorbed from blood circulation into the heart muscle cells followed by β-oxidation in these cells. Any mismatch between their entrance and consumption in the cardiomyocytes leads to lipotoxicity. Following lipotoxicity, myocytes show cellular dysfunction, signal transduction alteration, and apoptosis (6).
FAs have been classified into saturated fatty acids (SFA) and unsaturated fatty acid (UFA). Different effects of these two categories on muscle cells have been revealed. Palmitic acid, as an SFA, is one of the major sources of energy production in cardiomyocytes. However, any imbalance between their entrance to the cell and consumption results in the higher concentrations of palmitate in the cardiomyocytes which will enhance the production of ceramide and Diacylglycerol (DAG) (7, 8).
Previous studies reported that the accumulation of palmitic acid in cardiomyocytes (7, 9), pancreatic β-cells (10) and hepatocytes (11) caused lipotoxicity and disturbed cell functions. Beneficial effects of palmitoleic acid, the n-7 fatty acid, on antioxidant power and signal transduction alteration have been well documented (10, 12, 13). Lipotoxicity is a phenomenon known as the rebellious outcome of the accumulation of extra lipids in non-adipose tissues. We select rat cardiomyocytes as a non-adipose tissue. Considering the antioxidant effects of palmitoleic acid on various biological systems and the lack of reputable studies over its role in oxidative stress, the present study was conducted to investigate the effect of palmitoleic acid on the oxidative stress indices followed by palmitic acid-induced lipotoxicity in adult rat cardiomyocytes.
2. Methods
2.1. Preparation of Adult Rat Cardiomyocytes
Ventricular heart muscle cells were isolated from 300 - 350 g male Wistar rats as described previously (14) with a slight modification. The animal care committee of Urmia University approved the experimental design and sampling. In brief, 500 international unit heparin sulphate was injected into peritoneum 10 minutes before anaesthetization with chloroform. After opening the thorax and removing the heart, it was soaked into a petri dish filled with ice-cold physiological sodium chloride solution. The heart was connected from aorta to a perfusion system containing carbogenated perfusion buffer for 3 - 5 minutes. Perfusion continued for 20 - 25 minutes with 0.05% carbogenated trypsin and 0.02% EDTA in perfusion buffer. In the next step, ventricles were separated from the atriums and were cut into small pieces followed by digestion with trypsine-EDTA in perfusion buffer. Cell suspension was filtered through a mesh (with size of 200 µm) and then, it was centrifuged for 6 min at 25 g. The myocyte-containing pellet was re-suspended in the perfusion buffer by adding 0.2%, 0.4%, and 1% CaCl2 (v/v), respectively, and it was centrifuged again as before. Eventually, the pellet was re-suspended in bovine serum albumin (BSA) gradient (perfusion buffer, 4% BSA, 1% CaCl2) and re-centrifuged at 15 g for 5 minutes. Ventricular myocytes were seeded into 24-well-plates (about 100,000 cells per well) for 6 hours. Afterwards, the wells were washed with PBS and then, the experimental media containing 0.5 mM BSA-conjugated fatty acids were added. In this study, there were 4 groups including control, palmitic acid, palmitoleic acid, and palmitic+ palmitoleic acids (0.5 mM of each fatty acid was added). Cells were incubated for 48 hours after treatment. Variables were measured at 24h and 48h time points. Cell culture medium consisted of medium 199 (Sigma-Aldrich; cat. no. M5017) enriched with 5 mM creatine, 2 mM L-carnitine, 5 mM taurine, 100 units/ml penicillin, and 100 µg/mL streptomycin.
2.2. Determination of Total Cell Protein
Total cell protein was evaluated based on Bradford protocol (15). Briefly, after preparing Bradford reagent and stock solution of BSA, 10 serial concentrations (10 μL to 100 μL ) of BSA (1 mg/mL) were prepared and their volume was equalized to 100 μL with NaOH. Then, 100 μL of the prepared sample (99 μL deionized water + 1 μL cell extract) and 500 μL Bradford reagent were added to the tubes. Finally, the absorbance of the test tubes was measured using a spectrophotometer (Shimadzu, Japan) at 595 nm after 10 - 15 minutes. The absorbance of the samples was analyzed according to the standard plot.
2.3. Determination of Malondialdehyde (MDA) Concentrations in the Cells and Media
Malondialdehyde concentrations were measured according to the Fredrick method with some modifications (16). In brief, 500 μL media were added to 1 mL thiobarbituric acid (TBA) solution, completely mixed and then, 500 μL distilled H2O was added to 1 mL TBA solution and thoroughly shaken. Each tube was heated in boiling water bath for 15 minutes, then cooled and centrifuged for 10 minutes at 1000 g. The absorbance of supernatant was read at 532 nm wave length.
2.4. Determination of Total Antioxidant Capacity (TAC) in the Cells and Media
TAC values were measured according to Koracevic et al. with a slight modification (17). Briefly, 10 μL of the sample was added to the test tubes (A1 & A0 ) and 10 μL Uric acid to the standard tubes (UA1 & UA0 ); then, 490 μL PBS solution (0.1M PH, 7.2) was added to the test and standard tubes while 500 μL was added to the control ones (K1 & K0). PBS contained 600 mL dH2O + 84 mL NaH2PO4 0.2M + 216 mL Na2HPO4 0.2 M + 3.5 g sodium chloride. Afterwards, 500 μL Na-benzoate to all tubes and 1000 μL acetic acid to A0, K0, and UA0 tubes were added. Also, 200 μL Fe-EDTA followed by H2O2 were added to all tubes. The tubes were then incubated for 60 minutes at 37°C. Later, 1000 μL acetic acid to A1, K1, and UA1 tubes and 1000 μL TBA to all tubes were added. Finally, they were incubated for 10 minutes at 100°C in boiling water bath; they were then cooled and the absorbance of the samples were measured via a spectrophotometer (Shimadzu, Japan) at 532 nm wave length.
2.5. Statistical Analysis
All values were expressed as mean ± standard error of mean. The significant differences between MDA and TAC values of the experimental groups were assessed using one-way ANOVA in SigmaStat software (version 3.1). P < 0.05 was considered statistically significant.
3. Results
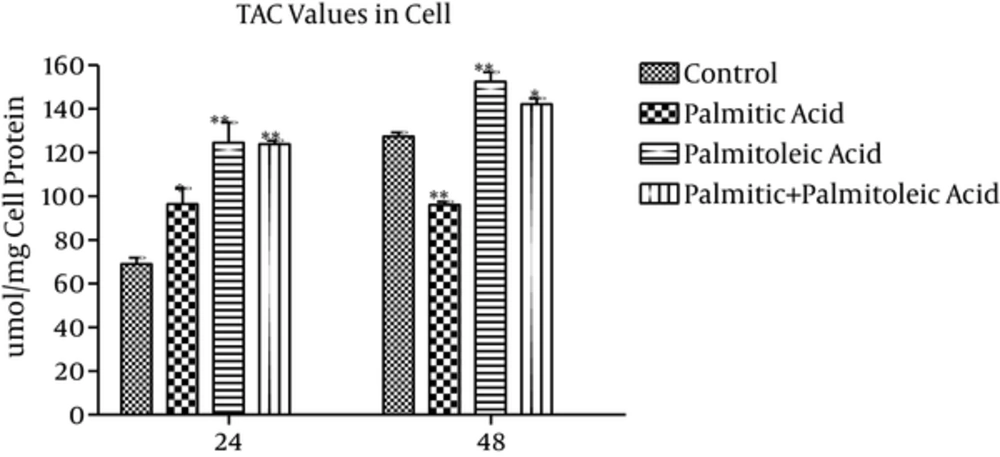
3.1. TAC values in the Cells
TAC values of the cells (μmol/mg cell protein) showed 29% (P = 0.004), 45% (P < 0.001), and 44% (P = 0.001) increase in palmitic acid, palmitoleic acid, and palmitic acid + palmitoleic acid-treated groups compared to the control group at time point 24h, respectively (Figure 1). While the highest increase was observed in palmitoleic acid-treated group in comparison with the control group (45% and 19% increase at 24h and 48h time points, respectively, P ≤ 0.001), the palmitic acid-treated group indicated the highest decrease (24%) over 48h time point compared to the control group (P < 0.001 Figure 1). Moreover, 11% increase was observed in TAC values of palmitic acid + palmitoleic acid-treated group at 48h time point (P = 0.02). Significant differences were observed between palmitoleic acid and control groups at 24h and 48h time points (P ≤ 0.01; Figure 1). In addition, considerable differences were noticed between palmitoleic and palmitic acids-treated groups during the experiment (P < 0.05; Figure 1). The TAC values of palmitic + palmitoleic acid group indicated a significant increase compared to the palmitic acid group over 24h (P = 0.048) and 48h (P < 0.001) time points (Figure 1).
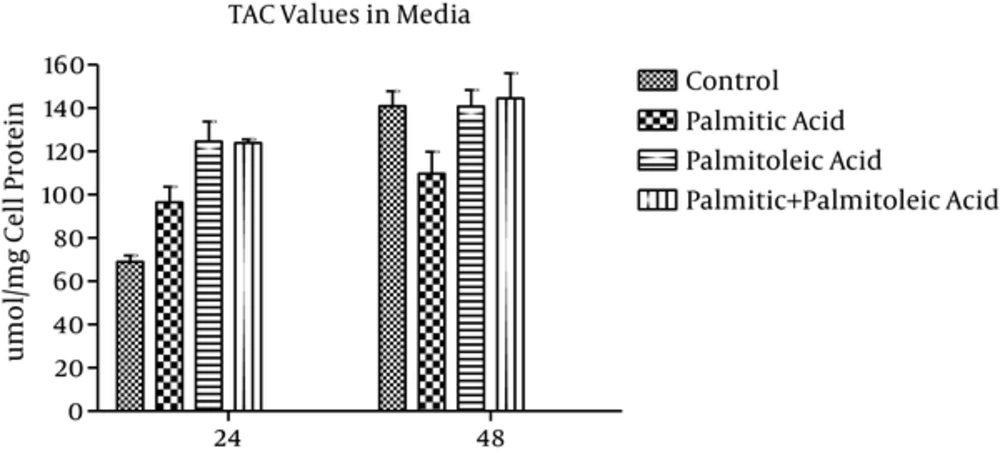
3.2. TAC Values in the Media
TAC values of the media (μmol/mg cell protein) showed 25% and 22% decrease in palmitic acid-treated group in comparison with the control group over 24h and 48h time points, respectively (P > 0.05; Figure 2). Additionally, palmitoleic acid-treated group showed 18% and 2% increase in TAC values compared to the control group at 24h and 48h time points, respectively (P > 0.05; Figure 2). Moreover, 26% and 2% increase in TAC levels of palmitic acid + palmitoleic acid-treated group was observed when compared to the control group (P > 0.05; Figure 2). While palmitoleic acid-treated group showed 18% increase in TAC values compared to the control group at time point 24h (P > 0.05), palmitic acid-treated group indicated 25% decrease (P > 0.05; Figure 2). Furthermore, TAC values increased in palmitoleic acid and palmitic + palmitoleic acid-treated groups compared to the palmitic acid-treated group during the experiment. These differences were not statistically significant (P > 0.05) except the difference between palmitic acid-treated group and palmitic + palmitoleic acid-treated group over 24h time point (P = 0.029; Figure 2).
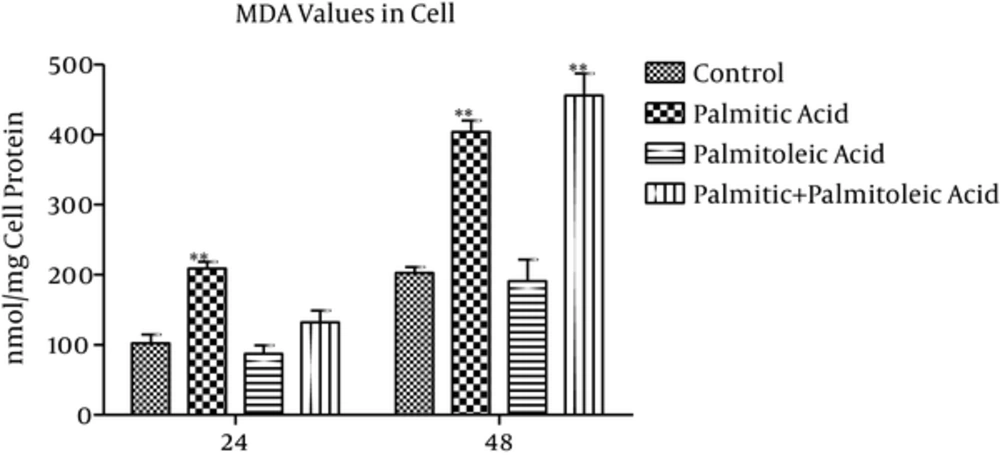
3.3. MDA Concentration in the Cells
MDA values of the cells (nmol/mg cell protein) showed 103% and 99% increase in the palmitic acid group compared to the control group at time points 24h and 48h, respectively (P = 0.002; Figure 3). Palmitoleic acid-treated group indicated 14% and 5% decrease in MDA values compared to the control group over 24h (P = 0.847) and 48h (P = 0.9) time points, respectively (Figure 3). The differences between palmitic acid and palmitoleic acid groups were significant at time point 24h (P < 0.001). Also, palmitic+ palmitoleic acid-treated group showed 29% and 125% increase in MDA values compared to the control group at 24h (P = 0.408) and 48h (P < 0.001) time points, respectively (Figure 3).
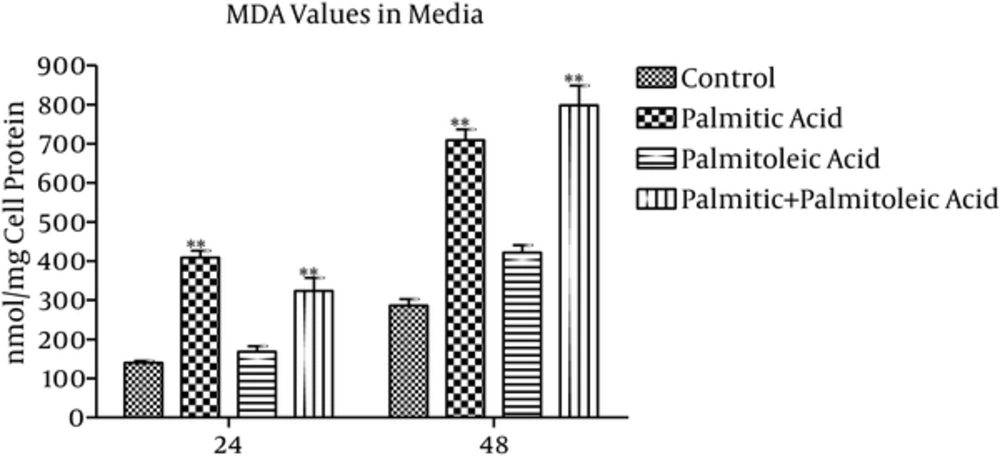
3.4. MDA Concentration in the Media
MDA values of the media (nmol/mg cell protein) showed 193% (P < 0.001) and 147% (P < 0.001) increase in the palmitic acid group, 20% (P = 0.766) and 47% (P = 0.059) increase in the palmitoleic acid group, and 132% (P < 0.001) and 179% (P < 0.001) increase in the palmitic + palmitoleic acid group compared to the control group over 24 and 48 time points, respectively (Figure 4). The differences between palmitic and palmitoleic acid groups were significant at time point 24h (P < 0.001; Figure 4).
4. Discussion
According to the previous investigations on antioxidant effect of monounsaturated fatty acids (MUFAs), it has been reported that HDL rich in oleic acid was less susceptible to oxidation (18) and the oral administration of oleic acid in adult rats could prevent oxidative stress in liver through inhibiting lipid peroxidation and improving enzyme activities (19). Also, some earlier studies have reported the beneficial effects of n-3 and n-6 polyunsaturated fatty acids on oxidative stress and antioxidant capacity (20, 21). However, some other researchers have revealed that n-3 FA available in fish oil not only could not significantly reduce antioxidant parameters in cardiomyocytes, but also enhanced MDA plasma concentrations in the athletes (22, 23).
The protective effects of palmitoleic and oleic acids have been reported in neonatal and adult rat cardiomyocytes (7, 8) and hence, our results are in line with the mentioned studies. In the present study, palmitoleic acid increased TAC values of cardiomyocyte at 24h and 48h time points in comparison with the palmitic acid and control groups in both the cells and the media, which could probably be due to the increased parameters contributing to antioxidant defense. Previous studies showed that the increase in rat cardiomyocyte antioxidant indices following treatment with palmitoleic acid could be a result of its potency to increase essential enzyme antioxidants like catalase, superoxide dismutase, and glutathione peroxidase (24). In addition, it was indicated that MUFA could strengthen cardiomyocytes antioxidant defense and PI3 kinase levels, which is one of the important factors in the viability of cardiomyocytes (25). In this regard, the mitochondria could be considered as the main organelle for oxidative damage and the major source of oxidant production. It is suggested that palmitoleic acid may hinder the SFA effects on electron transport chain reactive oxygen species production and might promote generation of complex I and III (4). Since cell adaptation in culture could result in an increase in TAC levels, it seems that the healing processes which sometimes follow the injuries made by culturing procedure can be related to these TAC up-regulation changes as seen in palmitic acid and palmitic acid + palmitoleic acid-treated groups compared to the control group. In this regard, several investigations have shown that oxidative stress condition caused increased antioxidant defense parameters in lymphoma cells (25). Thus, the mentioned increasing changes in these two groups could occur as a result of antioxidant defense stimulation during cell culture procedure.
In the present experiment, TAC values of palmitic group increased after 24 hour incubation compared to the control group. In this respect, Messier et al. reported that the TAC levels of mammalian cells increased after culture adaptation (26). It seems that cell adaptation is associated with the increase in TAC levels. Then, the increased TAC values of palmitic group after incubation might be related to noticeable stresses in the cells following cardiomyocyte culturing method. In the present study, palmitic acid-treated group also showed an increase in TAC values at 48h time point in cell and at 24h and 48h time points in the media compared to the control group, which is in line with the previous investigations retrieved from a research on neonatal and adult rat ventricular cardiomyocyte (7, 8). Moreover, the results of the present study have shown acceptable agreement with results of studies conducted on lipotoxicity in neonatal mice fibroblasts (27), human fibroblasts (28), rat proximal tubular cells (29), T lymphocytes, human aortic cells (30), and ß pancreatic cells (31).
One of the major products of lipid peroxidation is MDA, which is the polymerized cross linkage of nucleic acid, proteins, and some macromolecules. MDA content of the cells was evaluated as the lipid peroxidation index. It indirectly reflects the intracellular reactive oxygen species generation. In this present experiment, MDA concentrations in palmitoleic acid-treated group indicated a significant decrease compared to palmitic acid-treated group at 24h and 48h time points. However, co-administration of palmitoleic acid and palmitic acid did not show sufficient capability to avert palmitic acid lipid peroxidation activity. Moreover, the increased MDA concentrations in palmitic acid-treated group at 24h and 48h time points (intervals) may imply that the positive effect of palmitoleic acid solitarily on TAC can be a result of its capacity to hinder the oxidation of other products such as proteins. It was revealed that palmitic acid could oxidize the proteins in mice skeletal muscle cells (32) and in human skin fibroblasts (33). All of these facts strengthen this hypothesis that palmitoleic acid would prevent harmful effects of palmitic acid on TAC levels in lipotoxicity, affecting cardiomyocytes. In essence, the enzymatic defense including aconitas, catalase, and glutathione peroxidase can be altered by the active nitrogen generation due to mitochondrial protein modification using oxidant agents such as SFAs. It is suggested that palmitoleic acid may increase the amount of mentioned enzymes during antioxidant defenses. Hence, it seems to be essential to investigate the antioxidant enzymes’ activities and their gene expression (34).
4.1. Conclusions
It would be concluded that co-administration of palmitoleic acid and palmitic acid could reduce the harmful oxidant effects of palmitic acid. Further investigations are mandatory to find the effective dosage of these two fatty acids.