1. Background
Lead acetate is a well-known toxic heavy metal with potential detrimental effects on various organ systems, including the reproductive system. It has been associated with adverse effects on male reproductive health in both animal studies and human populations (1). While some studies have investigated the impact of lead acetate on general reproductive parameters in animals, its specific effects on sperm quality and concentration in male Wistar rats remain understudied. Understanding the potential reproductive toxicity of lead acetate is essential due to its wide industrial usage and environmental presence (2).
Sperm quality, encompassing morphology, motility, viability, and total sperm count, is a fundamental determinant of male fertility (3). Altered parameters in any of these aspects can significantly impact male reproductive health and fertility outcomes. For instance, abnormal sperm morphology can impede egg penetration, leading to reduced fertilization rates. Poor motility hinders sperm's ability to reach and fertilize the egg successfully, while low viability reduces the chances of healthy sperm reaching the egg. Research has established a strong correlation between sperm quality and fecundity, highlighting the vital role of comprehensive semen analysis in assessing male fertility potential and guiding appropriate infertility treatments (4). Investigating the effects of lead acetate on these sperm parameters is crucial for comprehending its impact on male fertility (5).
While research on lead acetate's impact on sperm quality in Wistar rats specifically is limited, studies conducted on other animal models suggest that lead acetate can lead to impaired sperm function and reduced fertility (6). Considering the similarities between different rodent models, it is reasonable to expect that male Wistar rats might also exhibit adverse effects on sperm parameters following lead acetate exposure.
Lead acetate's potential reproductive toxicity and its impact on sperm quality and concentration in male Wistar rats necessitate further investigation. Understanding these effects can provide valuable insights into the mechanisms of lead acetate-induced reproductive damage and may aid in formulating strategies to safeguard male reproductive health.
2. Objectives
Given the widespread industrial usage and environmental contamination of lead acetate, reducing exposure to this toxic heavy metal is crucial for promoting male fertility and overall reproductive well-being.
3. Methods
3.1. Animal Model
Male Wistar rats (n = 30) weighing between 200 - 250 grams were obtained from the Animal House of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife. The animals were housed in standard laboratory conditions with ad libitum access to food and water.
3.2. Experimental Groups
The rats were randomly divided into three groups, with ten rats in each group. Group 1 served as the control and received 1 ml/kg body weight of distilled water orally daily for 28 days. Group 2 was exposed to lead acetate (Sigma-Aldrich, USA) at a dose of 60 mg/kg body weight orally daily for 28 days. Group 3 underwent a recovery period, where they were administered lead acetate at the same dose for the initial 28 days, followed by 1 mL/kg body weight of distilled water orally daily for another 28 days.
3.3. Spermogram
At the end of the experimental period, the rats were euthanized, and epididymal sperm samples were collected using the standard "swim-up" method. Sperm motility was assessed immediately from the epididymal sample (7). Sperm viability was evaluated using the eosin-nigrosin staining method. Total sperm count was determined using a hemocytometer. Sperm morphology was examined by fixing and staining the sperm samples, and at least 200 spermatozoa were assessed under a light microscope (8).
3.4. Statistical Analysis
Data were analyzed using GraphPad Prism 8™, and results were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by the Neuman-Keuls post hoc test was used to compare the differences among the groups. P-value less than 0.05 was considered statistically significant.
3.5. Ethical Considerations
The study was conducted in compliance with the guidelines of the Institutional Animal Ethics Committee. All procedures involving animals were performed according to ethical standards, ensuring the welfare and humane treatment of the rats throughout the study.
4. Results
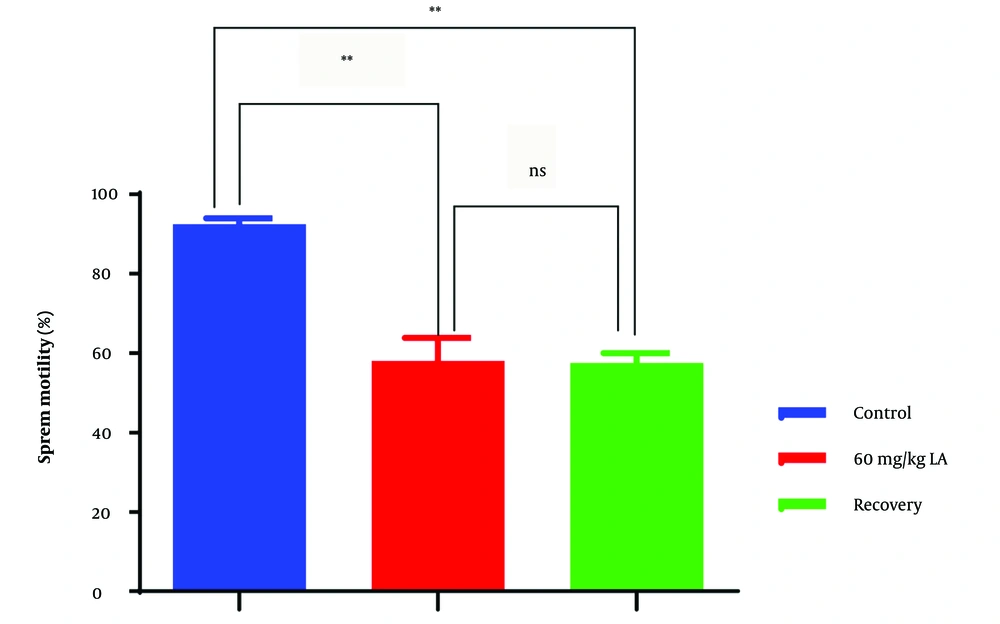
4.1. Sperm Motility
In the lead acetate-exposed group (group 2), sperm motility was significantly reduced compared to the control group (group 1) (Figure 1). This decrease in motility indicates impaired sperm movement and potential difficulties in reaching and fertilizing the egg. The lower motility observed in group 2 suggests that lead acetate exposure negatively impacts the functional capacity of sperm, which could lead to compromised male fertility.
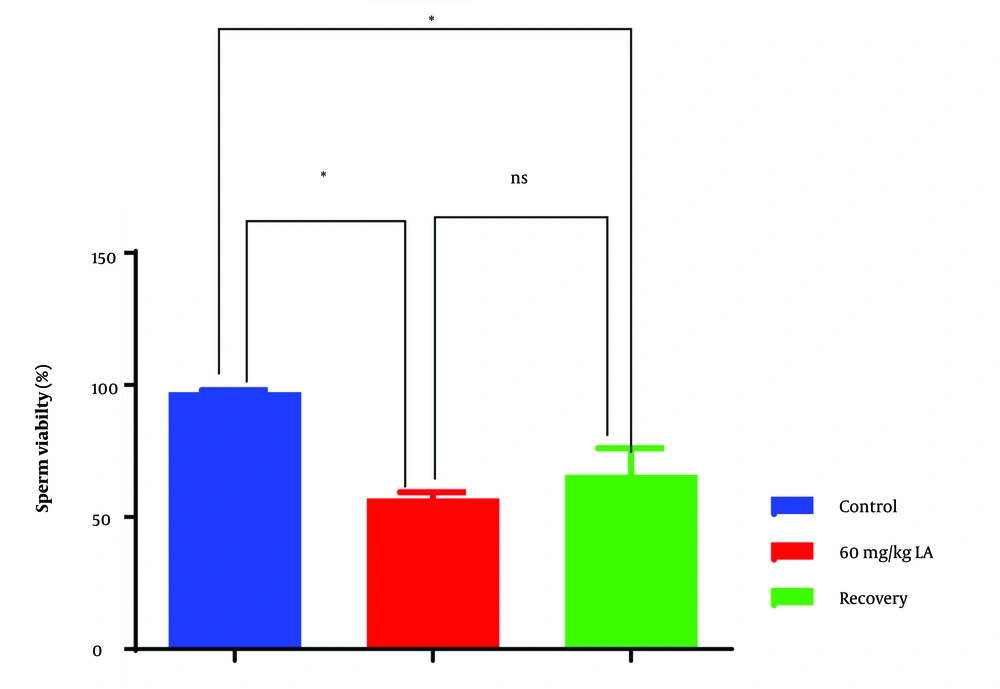
4.2. Sperm Viability
Group 2, exposed to lead acetate, exhibited significantly lower sperm viability compared to the control group (group 1) (Figure 2). Reduced sperm viability indicates a higher proportion of non-viable sperm, which are incapable of fertilizing an egg. This finding suggests that lead acetate exposure may lead to increased sperm cell death or decreased metabolic activity, contributing to compromised male fertility.
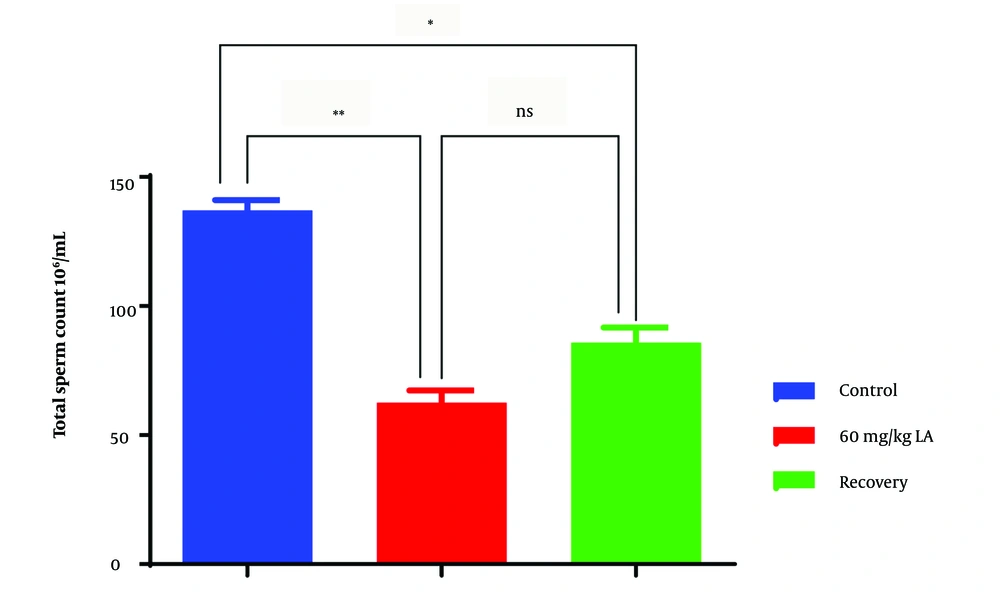
4.3. Total Sperm Count
The lead acetate-exposed group (group 2) showed a significant reduction in total sperm count compared to the control group (group 1) (Figure 3). A lower total sperm count indicates a decreased number of sperm available for fertilization. This observation suggests that lead acetate exposure may negatively impact sperm production or survival in the male reproductive tract, potentially leading to reduced fertility.
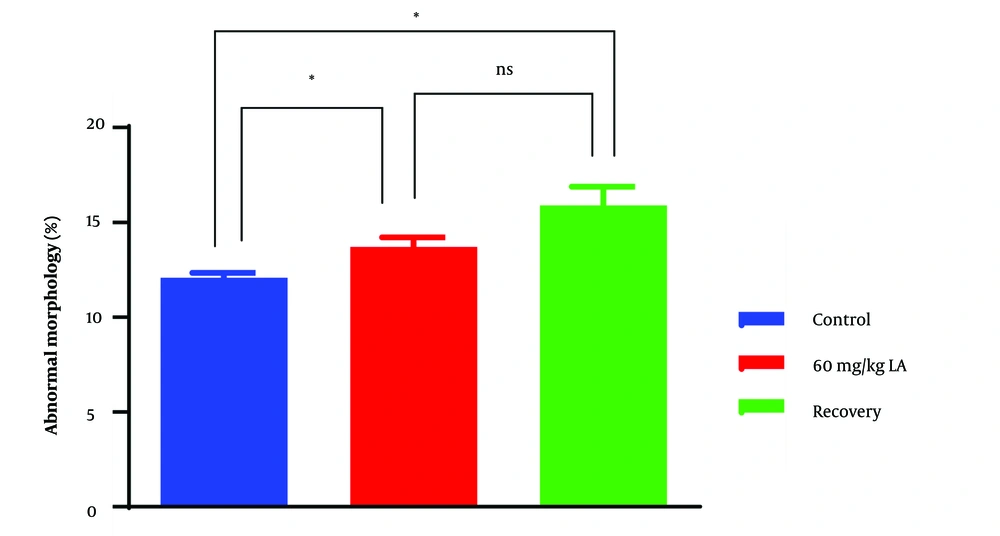
4.4. Sperm Abnormalities
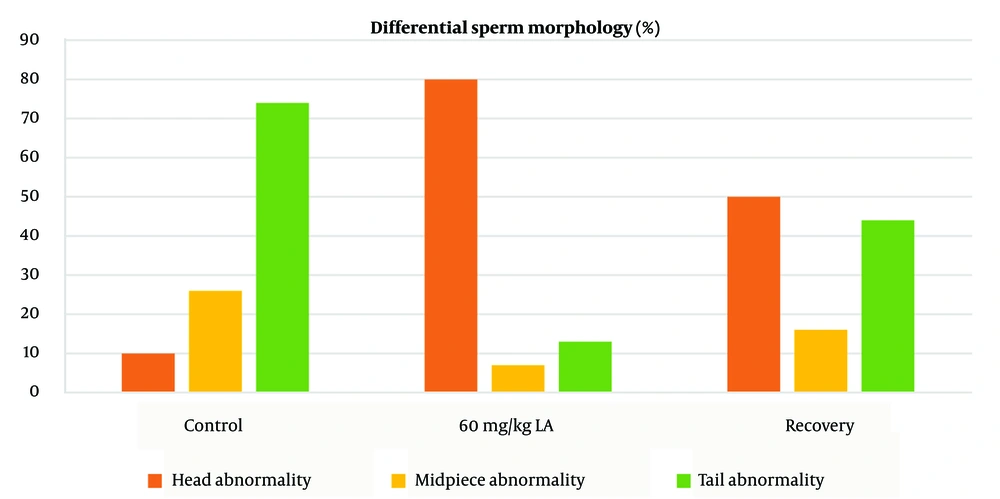
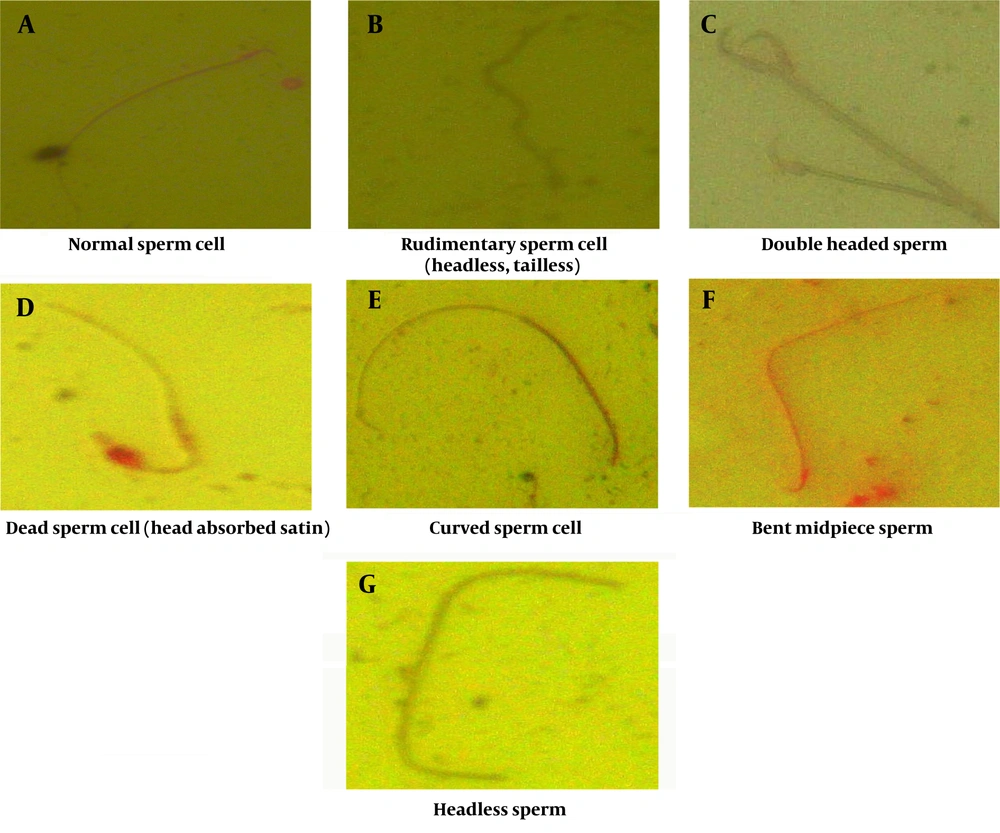
Group 2, exposed to lead acetate, demonstrated a higher incidence of sperm abnormalities compared to the control group (group 1) (P < 0.05) (Figures 4 and 5). Sperm head abnormalities, such as misshapen or damaged heads (Figure 6C, D, G), can impair sperm function and fertilization potential. The higher prevalence of sperm head abnormalities in group 2 indicates that lead acetate exposure may induce morphological changes in sperm, contributing to decreased male fertility.
4.5. Recovery Group (Group 3)
In the recovery group (group 3), which underwent a period of distilled water treatment after lead acetate exposure, some improvements in sperm parameters were observed compared to group 2. However, these improvements were not statistically significant. The lack of statistical significance suggests that the recovery period might not be sufficient to fully reverse the detrimental effects of lead acetate exposure on sperm quality and concentration.
Overall, the results indicate that lead acetate exposure adversely affects multiple sperm parameters, including motility, viability, total sperm count, and morphology. These findings highlight the potential reproductive toxicity of lead acetate and its detrimental impact on male fertility. The lack of significant improvements in the recovery group emphasizes the need for further research to explore effective interventions to mitigate the harmful effects of lead acetate on male reproductive health. Implementing measures to minimize lead acetate exposure and other environmental pollutants is crucial for safeguarding male fertility and overall reproductive well-being.
5. Discussion
The significant reduction in sperm motility observed in the lead acetate-exposed group suggests that lead exposure may interfere with the sperm's ability to move efficiently toward the egg during fertilization (9). Previous studies have shown that lead can induce oxidative stress, leading to the production of reactive oxygen species (ROS) that damage sperm membrane integrity and disrupt mitochondrial function, resulting in impaired motility (6, 7). Additionally, lead may disrupt calcium homeostasis in sperm, which is crucial for regulating motility patterns (10). Altered calcium levels can lead to abnormal flagellar movement, reducing sperm motility and contributing to male infertility.
The lower sperm viability observed in the lead acetate-exposed group may be attributed to lead-induced oxidative stress, leading to DNA damage and lipid peroxidation in sperm cells (10). Excessive ROS production can trigger apoptosis, leading to the premature death of sperm cells (11). Moreover, lead can interfere with the sperm membrane, compromising its structural integrity and rendering the sperm non-viable (12). The reduction in viable sperm cells may limit the pool of functional sperm available for fertilization, thereby reducing male fertility.
The significant decrease in total sperm count in the lead acetate-exposed group could be linked to lead's adverse effects on spermatogenesis (11). Lead exposure has been associated with disruptions in the hypothalamic-pituitary-gonadal axis, leading to decreased levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are essential for sperm production (7, 13). Furthermore, lead can accumulate in the testes and disrupt the blood-testis barrier, leading to increased germ cell apoptosis and reduced sperm production (14). These mechanisms may collectively contribute to the decline in total sperm count and subsequent fertility issues.
The higher incidence of sperm head abnormalities in the lead acetate-exposed group (Figures 5 and 6) suggests that lead may interfere with spermatogenesis and sperm maturation processes (15). One potential mechanism is the disruption of microtubule formation during spermiogenesis, leading to misshapen sperm heads (16). Lead's impact on calcium signaling in sperm can also disrupt acrosome formation, leading to abnormal acrosome development and, consequently, defective capacitation and acrosome reaction during fertilization (17). Abnormal sperm head morphology can hinder sperm-egg interaction, impairing fertilization and reducing male fertility (18).
The overall impact of lead acetate-induced abnormalities in sperm motility, viability, total sperm count, and morphology culminates in reduced male fertility (13). Sperm with impaired motility face challenges in reaching and penetrating the egg for fertilization. The decreased viability and total sperm count limit the number of viable sperm available for fertilization, decreasing the chances of successful conception. Additionally, sperm head abnormalities can negatively affect capacitation and the acrosome reaction, impairing the sperm's ability to undergo necessary changes for successful egg penetration.
5.1. Conclusion
This study reveals that lead acetate exposure severely impairs the reproductive health of male Wistar rats by reducing sperm motility, viability, and count, while increasing abnormalities in sperm head morphology. The detrimental effects are attributed to possible lead-induced oxidative stress and hormonal disturbances essential for spermatogenesis, as well as lead accumulation in the testes. These findings underscore the need for strategies to mitigate lead acetate exposure and further research into effective treatment methods to protect male fertility and overall well-being.