1. Background
Cardiovascular diseases are one of the most important problems in global health (1, 2). Various factors lead to cardiac and vascular disorders, especially genetic background, smoking, over weighting, hyperlipidemia, abnormal psychological conditions, and aging (3).
A large body of evidence shows that psychological risk variables are associated with physical diseases (4-7). Psychological risk factors, such as anxiety, depression, and stress can affect the cardiovascular system (3). As Hans selye described, stress refers to the consequences of the failure of a human or animal to respond appropriately to emotional or physical threats to the organism, whether actual or imagined (8). Thus, stress can disrupt the body’s homeostasis. Epidemiological studies have revealed that physical and psychological stress may be associated with depression (9). Variant data show the complex effect of stress or depression on body weight (10-12). Moreover, there is a reciprocal relationship between depression and cardiovascular disease (9).Chronic types of stress can induce dyslipidemia that promote atherosclerosis (13). Furthermore, previous studies have shown that stress causes arteriosclerosis yet the mechanisms of this effect are not well understood (9, 13, 14). It seems that stress could influence the hypothalamic-pituitary-adrenal (HPA) axis. This alteration could change the amount of stress hormones, such as catecholamines and corticosteroids. These hormones may trigger an inflammation reaction in the vascular system. The inflammation reaction influences both the vascular endothelium function and the recruitment of circulating monocytes and their conversion to foam cells. Overall, these changes generate structural disorders in vessels and promote arteriosclerosis (9, 13, 14).
Aging can influence vessels, as other parts of the body. Vascular aging is a process that is combined with different structural and functional changes in vessels during life. For example, intimal and medial thickening as well as gradual loss of arterial elasticity, results in vascular stiffness (15). Hardening of arteries in the elderly is often referred as arteriosclerosis (16).
2. Objectives
According to the high prevalence of senescence as a non-modifiable risk factor for vascular diseases and psychological risk factors as an inevitable part of the modern world, in this study, the researchers assessed the effects of chronic unpredictable stress on aorta structure, serum corticosterone, lipid level, and body weight in young and old rats.
3. Methods
3.1. Animals and Study Design
Thirty-two male Wistar rats (30 to 45 days old and six to seven months old) were obtained from the Pastor Institute of Tehran, Iran. The rats were housed in groups of four per cage and allowed to become adapted to their new environment for one week. The rats were divided to four groups (n = 8 in each group): young, young + stress, old and old + stress. Rats were weighed once a week during the course of the experiment for eight weeks. The final weight of rats was subtracted from initial weights to determine body weight gain over the course of the experiment.
Rats were maintained under a standard dark-light cycle (lights on between 8:00 am and 8:00 pm) with controlled ambient temperature (24 ± 2°C) and free access to standard food and water, except for the stressed groups during the period when the stressor applied required no food or water as well as disturbance on the dark-light cycle. Protocols were conducted in accordance with the standard ethical guideline, which was approved by the AJA University of Medical Sciences ethics committee.
3.2. Chronic Unpredictable Stress (CUS)
The CUS protocol used was a variety of unpredictable stressors previously described by Harro et al. (17) with slight modifications. Chronic unpredictable stress is compatible with depression in humans (5). Unstressed animals (young and old groups) were undisturbed, except for necessary procedures, such as weekly weighting and routine cage cleaning. However, stressed rats were exposed to the following stressors in a random order once a day between 8:30 and 12:30 am for eight weeks: cage tilting (45°C) for 24 hours, replacement of sawdust with sand for 24 hours, wet bedding for 24 hours, swimming in 4°C cold water for five minutes, exposure to 45°C for five minutes, overnight illumination, light off for three hours, 24 hours of food deprivation and 24 hours of water deprivation, tail pinch for one minute, isolation for 72 hours in a small cage, crowded housing for 24 hours, restrained for three hours.
3.3. Blood Sampling and Biochemical Assays
Fasting animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The chest wall was opened and blood samples were collected directly from the heart and centrifuged at 4000 × g for five minutes. The serum samples were collected and stored at -80°C until biochemical assays. Serum concentrations of triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), low-density lipoprotein to high-density lipoprotein ratio (LDL/HDL), very low-density lipoprotein (VLDL), and total cholesterol (TC) were measured by enzymatic assays using an automated spectrophotometric biochemical analyzer (Hitachi 912, Germany).
3.4. Tissue Harvesting and Processing
After blood sampling, the end part of the thoracic aorta was dissected and fixed in buffered formaldehyde and embedded in paraffin after standard dehydration steps. The paraffin blocks were sectioned (3 μm), and then stained with hematoxylin and eosin. Aortic thickness and inner diameter of aortic tissue were assessed by the ImageJ software.
3.5. Statistical Analysis
Statistical analysis was done using the SPSS version 18 software. Body weight gain, serum corticosterone, lipid levels and aortic tissue data, including aortic thickness and aortic diameter, were analyzed using two-way analysis of variance (ANOVA) with age as a between group factor and condition (i.e. control or stress) as a within group factor. The results are presented as mean ± standard error of the mean (SEM). P values of less than 0.05 were considered statistically significant.
4. Results
4.1. Effect of CUS on Body Weight Gain
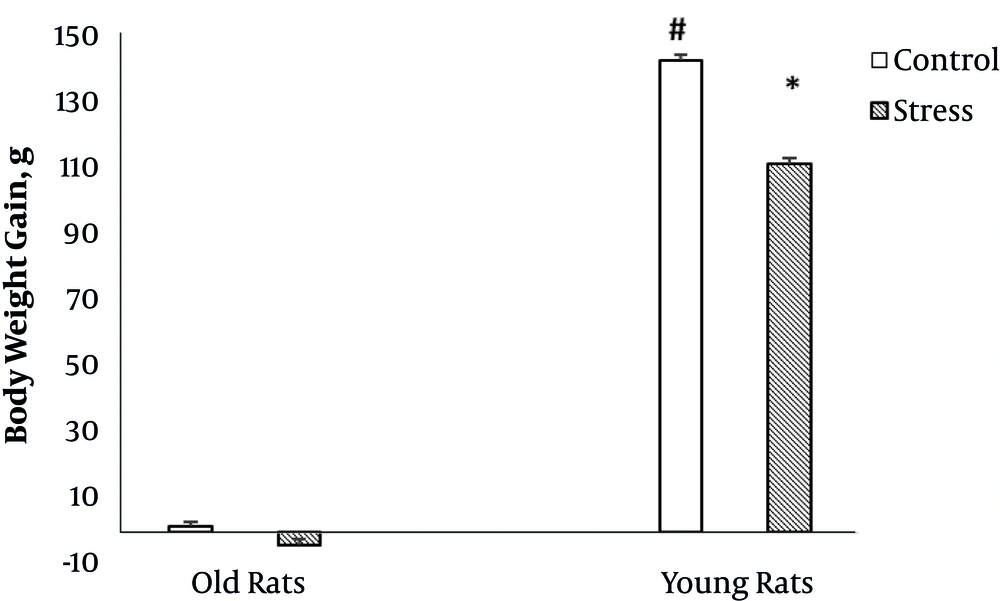
Figure 1 shows the effect of stress on body weight gain in old and young rats.
The results of two way ANOVA showed that age had a significant effect on body weight gain (F (1, 24) = 6001.3, P = 0.000). Also, there was a significant interaction between stress and age on body weight gain (F (1, 24) = 61.9, P = 0.000). However, in old rats, stress had no significant effect on body weight gain, yet in young rats, stress significantly decreased body weight gain.
4.2. Effect of CUS on Serum Corticosterone and Lipid Levels
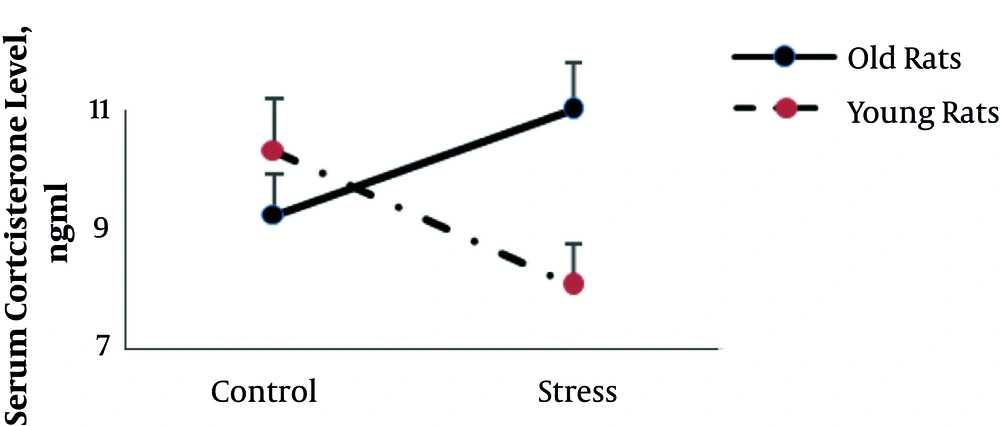
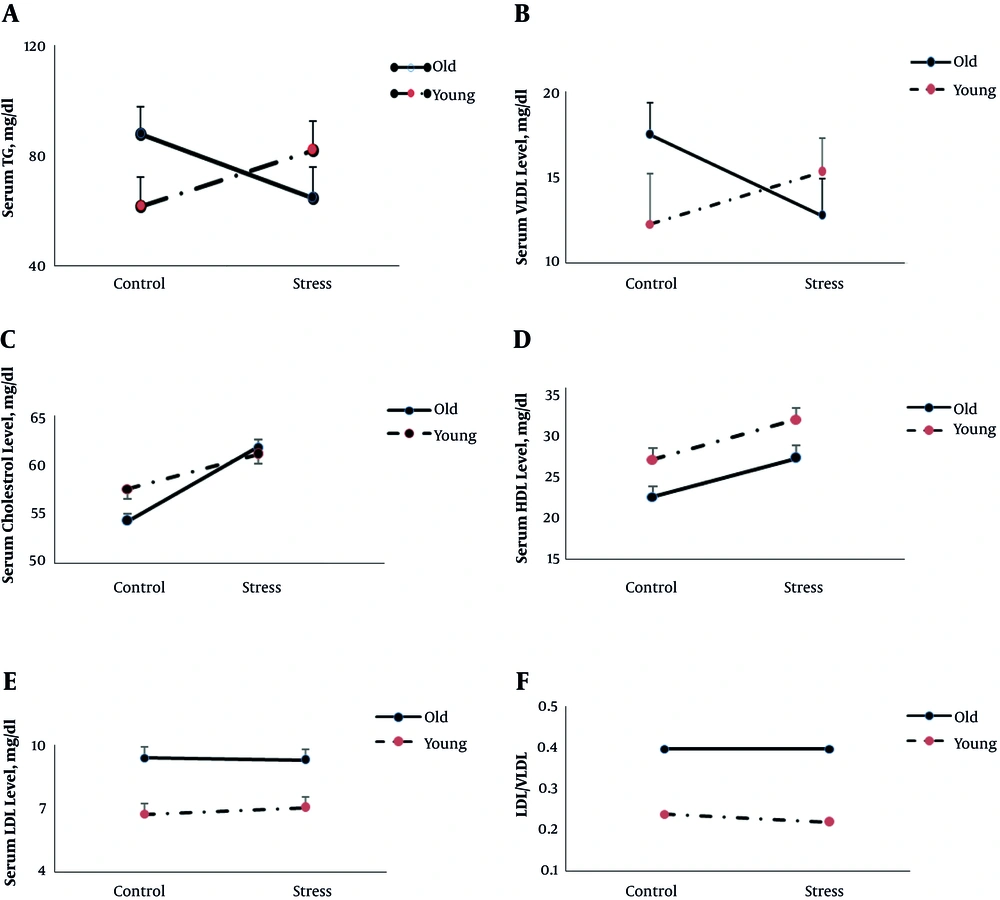
Figures 2 and 3 (A to E) shows the serum corticosterone and lipid concentrations in all groups, respectively.
The results of two-way ANOVA showed that age and stress had no significant main effect on serum corticosterone (P = 0.2 and P = 0.7 respectively), triglyceride (P = 0.6 and P = 0.8 respectively), and VLDL level (P = 0.6 and P = 0.8) yet there was a statistically significant interaction between the effects of age and stress on serum corticosterone (F (1, 22) = 7.4, P = 0.01), triglyceride (F (1, 24) = 4.4, P = 0.04), and VLDL level (F (1, 24) = 4.7, P = 0.04). However, in old rats, stress increases corticosterone and decreases triglyceride and VLDL levels; yet in young rats stress showed the opposite pattern. The results of two way ANOVA also revealed that stress significantly increased cholesterol level (F (1, 22) = 4.9, P = 0.03), yet there was no interaction between stress and age on serum cholesterol level (P = 0.4).
Age significantly affected LDL (F (1, 24) = 22, P = 0.000) and HDL (F (1, 24) = 10.6, P = 0.003) levels, and LDL/VLDL (F (1,24) = 30.5, P = 0.000).
Overall, in old rats, LDL (9.2 ± 0.3 mg/dL versus 6.9 ± 0.4 mg/dL) level and LDL/VLDL (0.4 versus 0.2) were higher and HDL level was lower (24.6 ± 1.1 mg/dL versus 29.6 ± 1.2 mg/dL) than young rats.
4.3. Effect of CUS on Histological Changes of Aorta
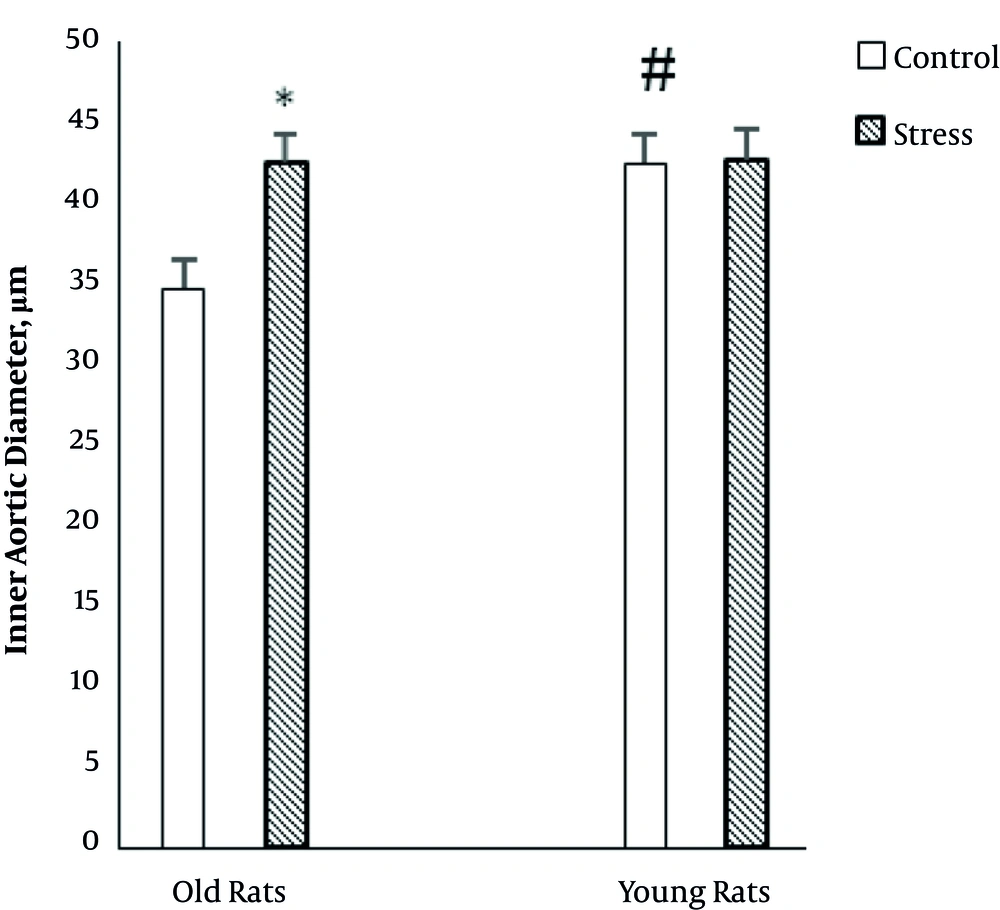
Figure 4 exhibits the effect of CUS on the inner diameter of the aorta in young and old rats. Two-way ANOVA showed that age (F (1, 24) = 5.45, P = 0.02) and stress (F (1, 24) = 5.6, P = 0.02) had a significant effect on the inner diameter of the aorta.
Also, there was a significant interaction between age and stress (F (1, 24) = 4.95, P = 0.03) on inner diameter of the aorta. The interaction effect indicates that the relationship between stress and inner diameter of the aorta depends on the age of rats. In older rats, stress significantly increases diameter of the aorta, yet in young rats, stress has not significant effect on the diameter of the aorta.
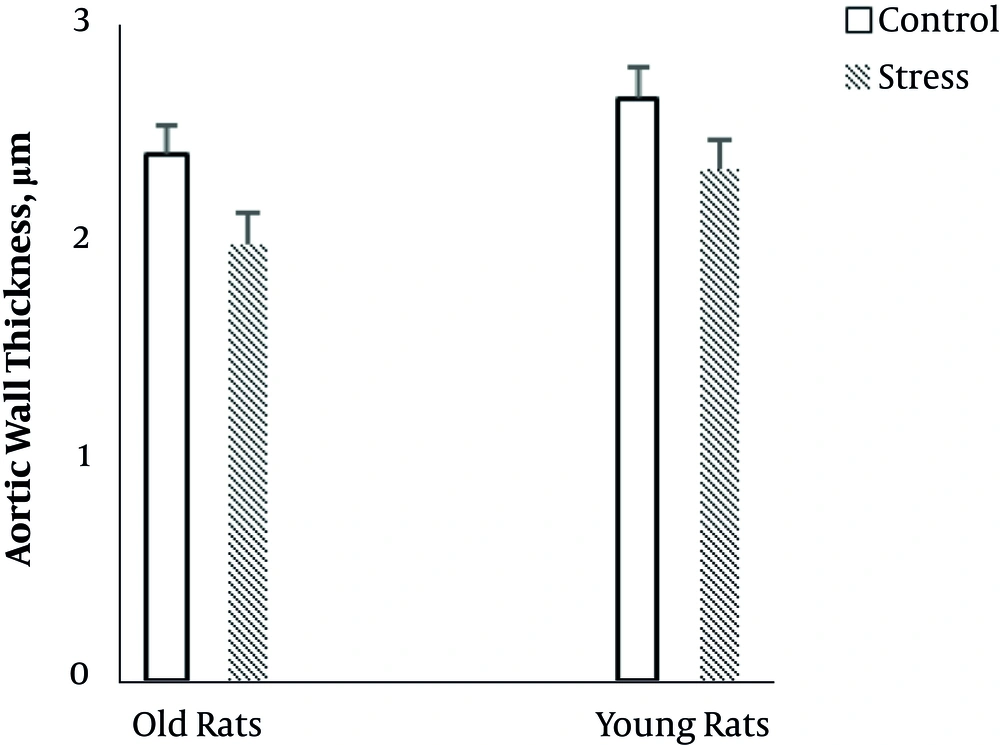
The results of two-way ANOVA showed that age (F (1, 23) = 4.7, P = 0.04) significantly influenced aortic wall thickness. Aortic wall thickness was more in young rats than old rats (Figure 5). Also, there was a significant main effect of stress (F (1, 23) = 6.64, P = 0.01) on aortic wall thickness. Stress decreased aortic wall thickness in old and young rats (Figure 5). There was no interaction between age and stress on aortic wall thickness.
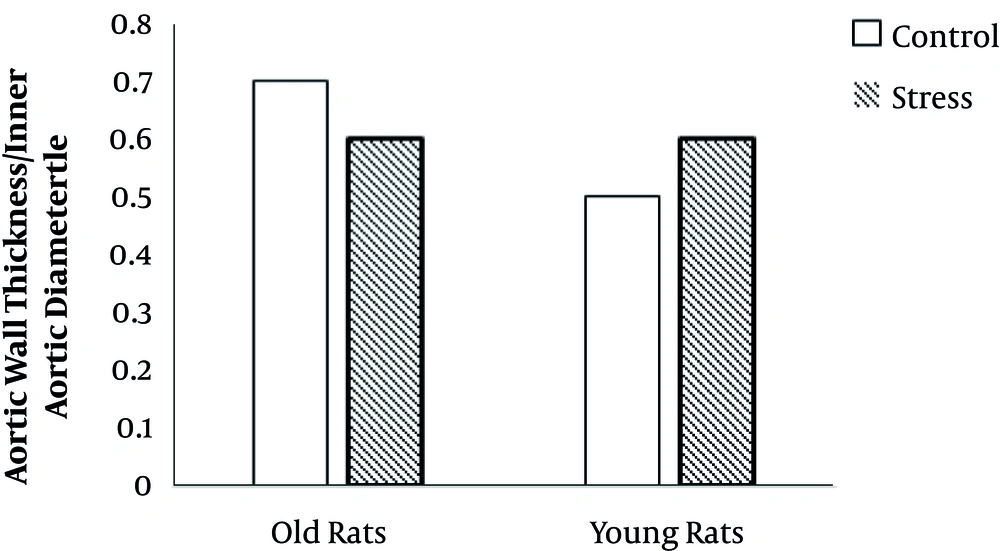
Age had a significant effect on aortic wall thickness/aortic inner diameter (AWT/AID) ratio (F(1, 23) = 11.1, P = 0.03). Stress had no main effect on AWT/AID. Also, there was no interaction between age and stress on AWT/AID (Figure 6).
5. Discussion
The relationship between psycho-physical stress and cardiovascular disease has been established (4, 9). Depression can be associated with psychophysical disorders (9). Likewise, aging is an unavoidable phenomenon that influences all systems of an organism, particularly, the cardiovascular system (16, 18-20). Due to the high probability of occurrence of both stress and aging together, in this study, the researchers investigated the effects of combined CUS and aging on body weight, serum lipids, and histological changes of aorta in rats.
The results of the current study showed that weight gain was significantly more in young rats than old rats. Also, CUS significantly decreased weight gain in young rats. Significant effect of stress on weight gain in young rats showed the reliability of the experimental model (5).
However, the body weight of old stressed rats did not show any significant difference compared to old control group, during eight weeks. It seems that old rats had stabilized body weight, yet young rats had possibility of weight gain through eight weeks for normal development, and CUS could restrain it.
In the current study, chronic stress in older rats, in contrast to young rats, significantly increased corticosterone concentrations. It seems that in older rats, the ability to adapt to stress and recovery has been reduced due to the reduction of the sensitivity of the brain and the pituitary to the inhibitory effects of corticosterone on the secretion of adrenocorticotropin secretion (21).
As expected, in this study, similar to previous studies (22-24), old rats showed higher LDL and LDL/VLDL levels and lower HDL levels than young rats. In the current study, two way ANOVA results showed that the effect of stress on some blood lipids was age-dependent. In old rats, stress reduced the concentration of TG and VLDL, and in young rats, it increased TG and VLDL levels. Previous studies have shown that elevated levels of triglycerides predict atherosclerosis and coronary artery disease (25), and VLDL along with LDLs contribute to atherosclerosis (26). Therefore, it seems that stress in old rats improves the level of some blood lipids that are known as “bad fats” and returns them to the level of the young group.
In contrast with most previous studies (27, 28), in the current study aortic wall thickness and inner aortic diameter in old rats was less than young rats and this result was somewhat surprising, yet aortic wall thickness/aortic inner diameter ratio was greater in old rats than young rats. This indicates that the effect of age on the thickness of the aortic wall was greater than the inner diameter of the aorta.
In this study, stress decreased aortic wall thickness in both (old and young) groups. A few studies have explored the effect of chronic stress on artery structure (29).
However, there are reports that chronic stress can cause cell apoptosis (30-32). Also, the study of Yu et al. (29) showed that chronic stress induces up regulation in the genes associated with apoptosis, and subsequently causes apoptosis in the vascular smooth muscle cells. Therefore, in the current study, reduction in wall thickness may be due to acceleration of apoptosis.
5.1. Conclusions
Blood lipid profiles were less desirable in elderly rats than in younger rats, yet in older rats, stress had more beneficial effects on lipid profile than younger rats. Aortic wall thickness/aortic inner diameter ratio was greater in older rats than younger rats. Stress decreased aortic wall thickness in both (old and young) groups.
More histological and biochemical investigations are needed to clarify the details of dual effects of CUS on vascular structures and plasma lipids in young and old rats.